Tau assemblies enter the cytosol of neurons in a cholesterol sensitive manner
Posted on: 7 July 2021
Preprint posted on 28 June 2021
Article now published in Cell Reports at http://dx.doi.org/10.1016/j.celrep.2022.110776
Tau aggregation in AD: Changes in cholesterol are shown to affect entry of tau into the cytosol in neurones and human cell lines.
Selected by Emma WilsonCategories: neuroscience
Introduction:
Alzheimer’s disease (AD) is the most common neurodegenerative disease in the world, contributing to approximately 50 million dementia cases worldwide. In AD, Tau, a major microtubule associated protein which promotes the assembly of tubulin into microtubules, becomes hyper-phosphorylated and aggregated into bundles1. This is also true for other related neurodegenerative diseases called tauopathies.
There are two main ways in which tau becomes aggregated within the diseased brain.
- Endogenous tau in each individual cell aggregates without endogenous tau.
- Tau can move in a prion-like fashion propagating from one cell to the next2.
It has been shown that the spread between cells may occur by uptake of naked tau, or through membrane-bound vesicles during endocytosis or micropinocytosis. However, how tau is released from intracellular vesicles into the cytosol is relatively unknown. It has been shown that low-density lipoprotein receptor Lrp1 and Heparan sulphate proteoglycans (HSPGs) are involved with tau seeding3,4 but how these may effect tau entry into the cytosol is yet to be known. In this preprint, Tuck et al. designed a novel assay to study entry of tau into the cytosol and established a role for the endocytic proteins clathrin and dynamin, as well as cholesterol, in the tau entry process.
Tau entry assay
The authors developed a highly sensitive method for the detection of tau entry into the cytosol. This assay can be conducted in live cells and relies on a split luciferase system. This system is composed of 11 amino acid fragments of luciferase, HiBiT, which is fused to tau, and a cytosolic LgBiT fragment.
When tau-HiBiT enters a cell through endocytosis it is unable to fuse with cytosolic LgBiT fragments until it is released from the intracellular vesicles. Upon release, the LgBiT can form a complete luciferase (NLuc), and with the addition of a substrate, luminescence can be measured. Thus, uptake of tau can be detected via measuring luminescence signal.
The authors found that the NLuc signal was concentration-dependent and increased proportionally to the amount of tau-HiBiT present in the cytosol. Thus, the authors expressed LgBiT by lentiviral transduction under the mammalian housekeeping promoter and with a nuclear localisation signal. This kept the cytosolic levels low to allow the authors to establish an assay that reports solely on cytosolic intracellular tau.
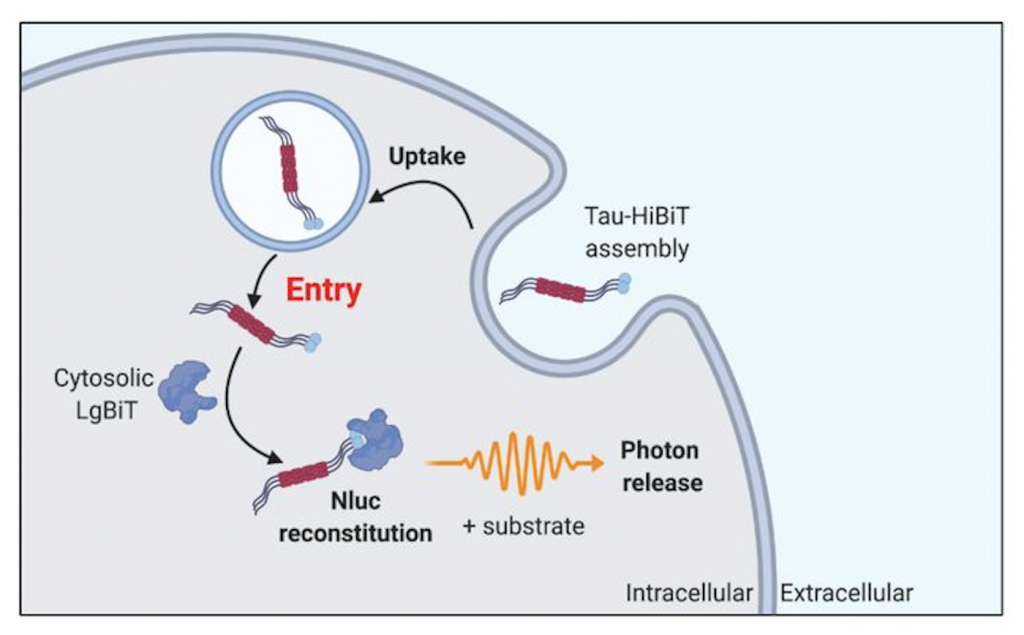
Key points of the paper
Tau uptake and entry into the cytosol of HEK cells is clathrin and dynamin dependent. However, this is cell-type dependent and does not happen in primary mouse neurons.
First, the authors transduced HEK293 cells with tau-HiBiT and inhibited key components of endocytic pathway, coat protein clathrin and GTPase dynamin, using inhibitors PitStop2 and Dyngo4a. The inhibition of these key endocytic protein resulted in reduced uptake of the fluorescently targeted transferrin, a key clathrin mediated endocytic cargo, and reduced entry of tau into the cytosol. This indicates that tau uptake and entry into the cytosol are clathrin and dynamin dependent.
Vacuole Protein Sorting 13 (VPS13) is a ubiquitin binding protein which has been shown to promote the seeded aggregation of tau through endolysosomal escape of tau seeds5. The authors depleted VPS13D in HEK293 cells, which resulted in an increase in tau entry into the cytosol. Similarly, a reduction in VPS35, known to be a genetic risk factor for Parkinson’s Disease and also linked to increased tau burden in late onset AD6,7, was correlated with a decrease in tau entry into the cytosol. These data suggest a role for endosome sorting machinery in preventing tau entry. Together, these data suggest that the endocytic and endosomal pathways are key regulators of tau uptake, entry to the cytosol and therefore tau seeding.
Next, the authors wished to study the mechanisms of tau entry in a more physiologically relevant system and so adapted their tau seeding assay to primary mouse neurons. LgBiT with nuclear localisation sequence and eGFP was expressed in primary mouse neurons using the human Synapsin promoter, a neuronal maker. Results showed that there was no change in entry of tau with the addition of clathrin and dynamin inhibitors, no difference to micropinocytosis, and no difference with knock-down of RAB GTPases in both Day 7 and Day 14 primary mouse neurons. This suggests that the entry of tau into the cytosol in primary mice neurons is mediated by a mechanism which is not clathrin or dynamin dependent.
Lrp1 and HSPGs facilitate the cholesterol dependent entry of tau into the cytosol of neurons
Lrp1 has recently been identified as a receptor for tau uptake4. The authors therefore wanted to investigate if Lrp1 has an effect on tau entry into cytosol in HEK293 cells. The depletion of Lrp1 corresponded to a decrease in tau entry into the cell 4h after the addition of LgBiT tau. In addition, Heparin, a ligand for HSPGs, cell surface receptors involved in many developmental signalling process and extracellular matrix protein, also reduced tau entry in a dose dependent manner suggesting a role of cell-surface HSPGs in promoting tau attachment.
Cholesterol has been associated with the accumulation of tau filaments in Neimann-Pick disease,8 and the Apolipoprotein (APOE) gene which encodes a cholesterol transporting protein, is also a genetic risk factor for AD9. Knowing this, the authors tested if cholesterol was needed for tau import by using both the cholesterol extracting agent methyl-beta-cyclodextin (MßCD) and cholesterol accumulating agent 25-HC. The extraction of cholesterol from the cell resulted in a significant increase in tau entry into the cytosol, while the accumulation of cholesterol in intracellular membrane bound organelles saw a significant reduction in cytosolic tau. This was also found to be reproducible in Organotypic hippocampal slice cultures10, where MßCD increased seeded aggregation while 25-HC reduced it. Together, this suggests that cholesterol plays a role in the entry of tau into the cytosol. It is thought that cholesterol alters the properties of the lipid bilayers, thus, decreasing cholesterol makes the membrane more prone to rupture and likely increases the entry of tau into the cytosol.
Why I chose this preprint
I chose this preprint because the authors have developed an extremely interesting new tool to study the prion-like movement of aggregated proteins found in neurodegenerative diseases. This has the potential to help investigate protein aggregation and seeding in many other neurodegenerative diseases linked to the accumulation of other proteins, such as alpha-synuclein in PD.
Questions to the author.
- Has this been tried with IPSC’s such as iNeurons which are a model of cortical neurons? Would you expect tau uptake to be similar to the human cell line or the mouse neurons?
- Could this assay be adapted for alpha-synuclein? Do you think it would have a similar mechanism?
- Why did you use split luciferase system rather than a split fluorescence protein system?
- Did you check uptake of transferrin or other cargo for each of the manipulations you did? Was there a decrease in uptake which could result in a decrease in entry into the cytosol when you knockdown VPS35?
- Are you planning to investigate uptake into the cell vs entry into the cytosol of tau?
- Are you interested in investigating early endosome genes BIN1, PICALM and CD2AP which are the most common polymorphisms associated with late onset AD? Would it be interesting to see their impact on the seeding assay?
References
- Goedert, M. Tau filaments in neurodegenerative diseases. FEBS Letters (2018). doi:10.1002/1873-3468.13108
- Sanders, D. W. et al. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron (2014). doi:10.1016/j.neuron.2014.04.047
- Holmes, B. B. et al. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc. Natl. Acad. Sci. U. S. A. (2013). doi:10.1073/pnas.1301440110
- Rauch, J. N. et al. LRP1 is a master regulator of tau uptake and spread. Nature (2020). doi:10.1038/s41586-020-2156-5
- Anding, A. L. et al. Vps13D Encodes a Ubiquitin-Binding Protein that Is Required for the Regulation of Mitochondrial Size and Clearance. Curr. Biol. (2018). doi:10.1016/j.cub.2017.11.064
- Vagnozzi, A. N. et al. VPS35 regulates tau phosphorylation and neuropathology in tauopathy. Mol. Psychiatry (2019). doi:10.1038/s41380-019-0453-x
- Williams, E. T., Chen, X. & Moore, D. J. VPS35, the retromer complex and Parkinson’s disease. Journal of Parkinson’s Disease (2017). doi:10.3233/JPD-161020
- Auer, I. A. et al. Paired helical filament tau (PHFtau) in Niemann-Pick type C disease is similar to PHFtau in Alzheimer’s disease. Acta Neuropathol. (1995). doi:10.1007/BF00318566
- William Rebeck, G., Reiter, J. S., Strickland, D. K. & Hyman, B. T. Apolipoprotein E in sporadic Alzheimer’s disease: Allelic variation and receptor interactions. Neuron (1993). doi:10.1016/0896-6273(93)90070-8
- Croft, C. L., Futch, H. S., Moore, B. D. & Golde, T. E. Organotypic brain slice cultures to model neurodegenerative proteinopathies. Molecular Neurodegeneration (2019). doi:10.1186/s13024-019-0346-0
doi: https://doi.org/10.1242/prelights.29943
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the neuroscience category:
Electrophysiological correlates of conscious experiences during sleep: Lucid dreams, sleep paralysis, out-of-body experiences, and false awakenings
uMontreal Neuro preLighters et al.
PPARδ activation in microglia drives a transcriptional response that primes phagocytic function while countering inflammatory activation
Isabel Paine
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
preLists in the neuroscience category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |











 (No Ratings Yet)
(No Ratings Yet)