Force requirements of endocytic vesicle formation
Posted on: 18 December 2020
Preprint posted on 11 November 2020
Article now published in Developmental Cell at http://dx.doi.org/10.1016/j.devcel.2021.08.007
Categories: biophysics
Background
Mechanical forces are key for many cellular processes, including clathrin-mediated endocytosis. This is the major trafficking pathway transporting nutrients, signals, pathogens and plasma membrane components into the cell. During endocytosis, forces provided by endocytic proteins, formation of multiprotein scaffolds, protein crowding, and polymerization of the actin cytoskeleton reshape the plasma membrane into a vesicle. To transmit forces stored in the actin polymerization and crosslinking, actin filaments must be mechanically linked to the plasma membrane. This is accomplished by the force-transmitting protein linker made of endocytic adaptors Sla2-Ent1 in yeast, and Hip1R-epsin 1-3 in humans. These proteins bind cooperatively to the plasma membrane by N-terminal domains, and redundantly to actin filaments through C-terminal domains. The absence of Sla2/Hip1R-epsin linker severely impairs endocytosis. Assessing force requirements of endocytic membrane remodeling is essential for understanding endocytosis. In their work, Abella and colleagues (1) aimed to measure forces applied on the Sla2-Ent1 linker during endocytosis in yeast, using calibrated FRET-based tension sensor modules (TSMs) integrated into Sla2 protein. Overall, this study provides key force values and force profiles for understanding the mechanics of endocytosis and potentially other cellular membrane-remodeling processes.
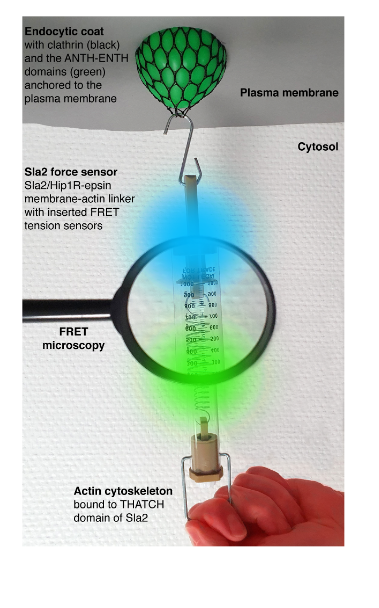
Key findings and developments
The authors began by constructing yeast strains expressing various Sla2 force sensors-Sla2 fusion proteins containing different tension sensor modules (TSMs, ref. 2) connected by mTurquoise2 and mNeonGreen fluorophores. The TSMs sensitive to distinct force ranges: F40 (1-6 pN), HP35 (6-8 pN) or HP35st (9-11 pN) were inserted between the coiled-coil dimerization motif and the actin-binding THATCH domain of Sla2. To distinguish force-dependent from force-independent FRET changes, the authors also constructed strains with all TSMs integrated after the THATCH domain, at the C-terminus of Sla2.
To follow the forces applied on the Sla2 force sensors, the authors analysed FRET changes during individual endocytic events- by simultaneously recording the mTurquoise2 and mNeonGreen fluorescence signals (i.e. FRET ratio) at individual endocytic sites. A decreased ratio between mNeonGreen and mTurquoise2 fluorescence intensity during endocytic events, indicated force applied on all 3 Sla2 force sensors. FRET ratio profiles of all three Sla2 force sensors showed an initial decrease in the FRET ratio approximately 13 seconds before vesicle scission. This coincided with the appearance of fluorescence signal of the actin marker Abp1-mScarlet-I at the endocytic sites, indicating that the force applied over the Sla2 sensors correlates with the onset of actin polymerization at the endocytic site. The similar starting point of the FRET ratio drop and its subsequent stepwise decrease observed for all 3 sensors suggests that Sla2 molecules are sequentially recruited to the growing actin cytoskeleton during the course of endocytosis. Comparing the 3 force sensors showed that while the Sla2-F40 and Sla2-HP35 sensors behaved similarly, a smaller decrease in FRET ratio was observed for the Sla2-HP35st sensor (suited to detect higher forces). This indicates that the force applied over Sla2 therefore lies inside the dynamic range of HP35st (around 10 pN). This value, connected with the number of Sla2 molecules at the endocytic site (45-133, refs. 3 and 4) results in 450-1300 pN transmitted over the Sla2 linkers during a single endocytic event.
The authors then analysed the contributions of key membrane-remodeling factors to endocytic force transmission using the Sla2-HP35 sensor. They began by following the FRET ratio during endocytic events in cells deleted for protein Rvs167 (BAR-domain protein known to bind membrane invaginations during endocytosis). In these cells, membrane invagination is sometimes aborted and the membrane retracts back to its initial flat conformation without vesicle scission. The authors conclude that similar forces are applied on the Sla2 linker in WT cells and in Rvs167 mutants during initial membrane bending and early invagination, but following this, Rvs167 is crucial to facilitate productive transmission during the later invagination steps. Next, the authors analysed the role of the organization of the actin cytoskeleton by following the Sla2-HP35 sensor in cells lacking Bbc1- a negative regulator of actin polymerization at the endocytic site. Analysis of changes in the FRET ratio suggests that less force is transmitted over Sla2 in the last phase of invagination. This led the authors to conclude that there is extra force stored in the actin cytoskeleton of bbc1-mutant cells, which directly remodels the invaginating membrane.
The authors went on to investigate the roles of turgor pressure and plasma membrane tension during endocytosis. The internal turgor pressure of yeast cells is the main mechanical barrier counteracting endocytic membrane invagination. To compensate for this the cells were placed in hypertonic medium to lower the osmotic difference between the cell cytoplasm and the surrounding environment. This resulted in a smaller drop in the Sla2-HP35 FRET ratio, suggesting that less force is transmitted over the sensor under conditions reducing cellular turgor. This was also observed upon incubating the cells with a soluble lipid palmitoylcarnitine, which is incorporated into the yeast plasma membrane and reduces its tension. Together, these results suggest that a decrease of cell turgor pressure or plasma membrane tension reduce the force required for endocytosis.
Finally, the authors analysed the capacity of the endocytic force-transmitting machinery, by following Sla2 force sensors in cells incubated under hypotonic conditions, which should intensify cell turgor opposing endocytosis. For this, they used mutant cells that lack aquaglyceroporin and thus cannot adapt to hypoosmotic conditions by glycerol efflux. These cells containing Sla2-HP35 or Sla2-HP35st sensors were exposed to osmotic shifts. As osmolarity was lowered, the number of stalled endocytic events increased. However, despite these differences in numbers of stalled events, no significant difference was detected in the force transmitted over the Sla2-HP35st sensor during completed endocytic events. This indicates that there is no significant adaptation of the force-generating and/or transmitting system to hypoosmotic conditions. Altogether, the authors hypothesize that under hypotonic conditions, yeasts maintain the vital endocytic process by modulation of internal turgor and not by direct regulation of endocytic force-generating or transmitting machinery.
What I like about this preprint
I found the topic very interesting and relevant for various fields. As the authors themselves point out, endocytosis is the major trafficking pathway for nutrients, signals, pathogens and plasma membrane components into the cell- which is relevant to a plethora of research fields. I found also the methods implemented by the authors very interesting and novel.
References
- Abella et al, (2020), Force requirements of endocytic vesicle formation, bioRxiv.
- Cost, A.L., Khalaji, S., and Grashoff, C. (2019). Genetically Encoded FRET-Based Tension Sensors. Curr. Protoc. Cell Biol. 83, 1–29.
- Picco, A., Mund, M., Ries, J., Nédélec, F., and Kaksonen, M. (2015). Visualizing the functional architecture of the endocytic machinery. Elife 2015, 1–29.
- Sun, Y., Schöneberg, J., Chen, X., Jiang, T., Kaplan, C., Xu, K., Pollard, T.D., and Drubin, D.G. (2019). Direct comparison of clathrin-mediated endocytosis in budding and fission yeast reveals conserved and evolvable features. Elife 8:e50749.
doi: https://doi.org/10.1242/prelights.26542
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the biophysics category:
Active flows drive clustering and sorting of membrane components with differential affinity to dynamic actin cytoskeleton
Teodora Piskova
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
Mitochondrial Fatty Acid Oxidation is Stimulated by Red Light Irradiation
Rickson Ribeiro, Marcus Oliveira
preLists in the biophysics category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
66th Biophysical Society Annual Meeting, 2022
Preprints presented at the 66th BPS Annual Meeting, Feb 19 - 23, 2022 (The below list is not exhaustive and the preprints are listed in no particular order.)
| List by | Soni Mohapatra |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
Biophysical Society Meeting 2020
Some preprints presented at the Biophysical Society Meeting 2020 in San Diego, USA.
| List by | Tessa Sinnige |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Biomolecular NMR
Preprints related to the application and development of biomolecular NMR spectroscopy
| List by | Reid Alderson |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |











 (No Ratings Yet)
(No Ratings Yet)