Spiral-eyes: A soft active matter model of in vivo corneal epithelial cell migration
Posted on: 3 September 2024 , updated on: 27 November 2024
Preprint posted on 18 August 2024
Unraveling the mysteries of corneal cell migration with active matter physics.
Selected by Prasanna PadmanabanCategories: biophysics
Background
When you blink or shed a tear, your cornea—the clear outer layer of your eye—quietly performs its job, keeping your vision sharp and your eyes healthy. Beneath the surface, though, there’s an intricate dance of cells working to keep the cornea functioning. But how exactly do these tiny epithelial cells move and organise themselves? This is where Kaja Kostanjevec and her colleagues step in with their fascinating new study.
What’s happening in our cornea? Our cornea is made up of several layers of cells, and the outermost layer – the epithelium is constantly renewing itself. These epithelial cells are crucial for wound healing and protecting the eye from injury or infection. But the way these cells move across the corneal surface is random. In fact, researchers have observed that the cells often migrate in striking spiral patterns. Why spirals? That’s one of the mysteries Kostanjevec and her team set out to solve using a multiphysics approach. In this preprint, the authors investigate the migration patterns of corneal epithelial cells using a soft active matter based computational model.
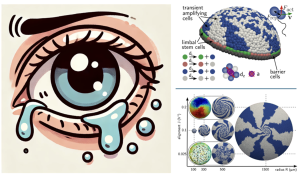
(Left) While shedding tears, the human eye undergoes a renewal process in the corneal epithelium, during which the corneal epithelial cells ‘dance’ in a coordinated fashion to form spiral patterns compensating for the cellular loss (cartoon generated using ChatGPT 4). (Right) computational models mimicking these processes (images reproduced from the preprint Figure 5 and Figure 7a, under CC-BY-NC 4.0 International license.)
Key Findings
Bringing Physics into Biology
The application of soft matter physics allowed the researchers to create a realistic and predictive model of corneal epithelial cell migration, showing that the cell’s intrinsic active properties, combined with the tissue’s geometry, are sufficient to drive the complex spiral patterns observed in nature. They employed a combination of in vivo imaging and computational modelling to describe a phenomenon that remained unexplored until now: the spiral migration of corneal epithelial cells. Overall, their findings provide insights into the biophysical mechanisms underlying corneal cell migration and highlight the potential of active matter models in studying complex biological processes.
Spiral patterns are not just for aesthetics
The authors simulated the spiral corneas by incorporating the forces generated by the cells as they move, called patterns. The model includes the mechanical interactions between cells, such as cell-cell adhesion and the forces exerted by cells on the surrounding tissue. These interactions are crucial for maintaining tissue integrity and influencing cell movement. A noteworthy aspect of the model is the incorporation of the cornea’s curved geometry. The curvature of the corneal surface influences how the cells align and move, contributing to the emergence of the spiral patterns observed in vivo. By simulating these elements together, the model demonstrates how individual cell behaviors and mechanical constraints can lead to the collective migration patterns seen in the cornea, particularly the formation of spiral structures.
Cellular alignment, velocity, and cornea radius are key drivers of spiral migration patterns
In their computational model, the authors simulated the properties of corneal epithelial cell migration based on established biophysical principles and experimental data. They adjusted parameters within the model to examine how changes in cellular alignment influence the direction of cell movement and force alignment. Cells naturally tend to align with their neighbors, moving collectively in a coordinated way, which contributes to the formation of spiral patterns. To better simulate cell movement dynamics, the model incorporated cellular velocity, which represents the speed at which individual cells move across the corneal surface. The researchers observed that variations in cellular velocity, driven by factors like cell-cell interactions and external signals, can impact the development and stability of these spiral patterns.
Another critical factor in the model is the curvature of the cornea, represented by the cornea radius. By adjusting the radius parameter to manipulate corneal curvature, the authors explored how different curvatures influence cell alignment and migration. They discovered that the curvature of the cornea plays a key role in guiding cells into spiral formations, with the radius determining the scale and tightness of these spirals. These findings highlight that cellular velocity, force alignment, cornea radius are essential to the organization and function of corneal epithelial cells, rather than being mere incidental elements.
Matter to Medicine
Understanding how corneal epithelial cells move isn’t just a matter of scientific curiosity—it has real-world applications. The work of Kostanjevec and her team opens the door to even more discoveries about how cells interact with their environment. When the cornea is damaged, whether from injury or disease, these cells need to migrate to cover the wound and restore the tissue. A deeper understanding of this migration could lead to better treatments for eye injuries and even improved techniques for growing tissues in labs. Their model could be adapted to study other tissues, especially those that exist on curved surfaces or have complex shapes. This could include everything from skin cells to cells in the gut lining.
By modeling the way corneal epithelial cells behave under normal conditions, scientists can also start to understand what might go wrong in diseases or during aging when cell migration becomes impaired. Beyond eye health, this work adds to finding new ways to explore the deep connection between physics and biology, and how these principles can be harnessed to better understand—and maybe even improve—how our bodies work.
What I like about this preprint
As we continue to dive deeper into the microscopic world of our cells, tools like soft active matter models will be increasingly important. This spiral-eyes study shows how innovative thinking at the intersection of disciplines can provide powerful new insights into biological processes that we’ve barely begun to understand – collective cell migration in the presence of curvature. So next time you blink, remember that there’s a beautiful, spiral-patterned dance happening in your cornea, keeping your eyes healthy and your vision clear.
Questions to the authors
(1) How comparable are the cornea of mice, to fishes and humans?
(2) What are your thoughts on using tissue chips to replicate cell migration events in the cornea? Do you think this could serve as an effective model to test your hypothesis?
(3) Which parameter is more crucial for predicting the healthy versus diseased state of corneal epithelial cell migration: cell shape or cell properties?
doi: https://doi.org/10.1242/prelights.38237
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the biophysics category:
Active flows drive clustering and sorting of membrane components with differential affinity to dynamic actin cytoskeleton
Teodora Piskova
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
Mitochondrial Fatty Acid Oxidation is Stimulated by Red Light Irradiation
Rickson Ribeiro, Marcus Oliveira
preLists in the biophysics category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
66th Biophysical Society Annual Meeting, 2022
Preprints presented at the 66th BPS Annual Meeting, Feb 19 - 23, 2022 (The below list is not exhaustive and the preprints are listed in no particular order.)
| List by | Soni Mohapatra |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
Biophysical Society Meeting 2020
Some preprints presented at the Biophysical Society Meeting 2020 in San Diego, USA.
| List by | Tessa Sinnige |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Biomolecular NMR
Preprints related to the application and development of biomolecular NMR spectroscopy
| List by | Reid Alderson |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |











 (1 votes)
(1 votes)