Responses to conflicting binocular stimuli in mouse primary visual cortex
Posted on: 28 October 2025 , updated on: 3 November 2025
Preprint posted on 1 September 2025
Categories: animal behavior and cognition, neuroscience
Background:
Binocular vision enables organisms to perceive a unified, three-dimensional view of the world by integrating two slightly different images from each eye. Under normal conditions, the visual system effectively fuses modest spatial disparities between retinal images to support depth perception, or stereopsis (Parker, 2007). This ability depends on the brain’s capacity to match corresponding visual features across the two eyes within a certain disparity range.
However, when disparities exceed this range either due to natural variance or pathological conditions such as strabismus, the visual system fails to achieve fusion, resulting in phenomena like diplopia (double vision) or binocular rivalry (Harrad et al., 1996; Blake & Logothetis, 2002). The neural mechanisms that underlie the transition from binocular integration to perceptual suppression under conditions of interocular conflict remain incompletely understood.
The authors utilized the mouse visual system to investigate how circuits in the primary visual cortex (V1) respond to qualitatively different types of binocular conflict. V1 is the first cortical stage where inputs from the two eyes converge, making it a critical site for binocular integration and disparity processing. They employed visual evoked potentials (VEPs), extracellular recordings, and two-photon calcium imaging to examine neural responses to two forms of interocular disparity: one in which the stimuli maintain the same orientation across both eyes but differ in phase, and another where the stimuli differ in orientation between the eyes, mimicking the conditions commonly used to study binocular rivalry. VEPs capture the summed electrical activity of neuronal populations in response to visual input, providing a population-level readout of how cortical responsiveness changes under different binocular conditions.
Key findings:
1. Binocular conflict suppresses cortical responses
When the two eyes viewed stimuli differing in phase or orientation, visually evoked potentials (VEPs) recorded from binocular V1 were markedly reduced, indicating that cortical responses are actively suppressed under interocular conflict. This suppression was evident even at low contrast levels, suggesting that V1 neurons detect and down-weight discordant binocular input early in the visual processing hierarchy.
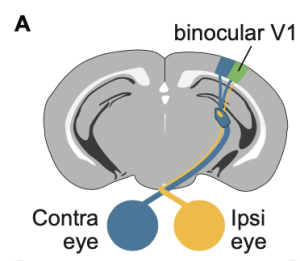
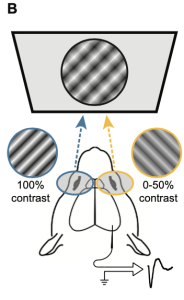
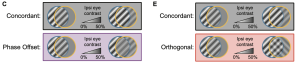
Figure 1. Binocular stimuli with an interocular phase or orientation disparity elicit smaller visually evoked potentials. (A) Schematic of mouse brain showing placement of a chronic recording electrode in binocular V1 (V1b). (B) Mice viewed phase reversing sinusoidal grating stimuli on a dichoptic display. (C) Phase offset experimental design. The contralateral (Contra) eye viewed full-contrast stimuli, while the ipsilateral (Ipsi) eye viewed stimuli ranging from 0% to 50% contrast, presented either in phase (Concordant) or out of phase (Phase Offset) with the contra eye stimulus. (E) Orthogonal orientation experimental design. Preprint figures placed in the Public Domain.
2. High sensitivity to subtle mismatches
Systematic variation of interocular orientation revealed that a 10° orientation difference between the eyes was sufficient to significantly attenuate VEP amplitude. This demonstrates that binocular V1 circuits are highly sensitive to even minor disparities, consistent with the role of early visual cortex in establishing precise interocular correspondence required for depth perception and binocular fusion.
3. Distinct neural mechanisms for different conflict types
Phase and orientation disparities recruited different temporal components of the VEP and distinct spiking dynamics. Phase-offset stimuli selectively suppressed the early negative VEP component and reduced early cortical spiking, whereas orthogonal stimuli diminished the later positive component but elicited prolonged activity across layers. These dissociable signatures indicate that V1 engages separate circuit mechanisms to process phase and orientation mismatches.
4. Layer- and cell-type–specific modulation
Laminar recordings revealed that conflict-related suppression and enhancement patterns depend on both cortical layer and neuronal subtype. Regular-spiking (excitatory) neurons showed early response suppression for phase-offset stimuli in layers 2/3, 5, and 6 but increased late activity under orthogonal stimulation. Fast-spiking (inhibitory) neurons exhibited extended activation primarily in layer 2/3 during orthogonal conflict. These results demonstrate structured, layer-specific reorganization of intracortical dynamics in response to binocular disparity.
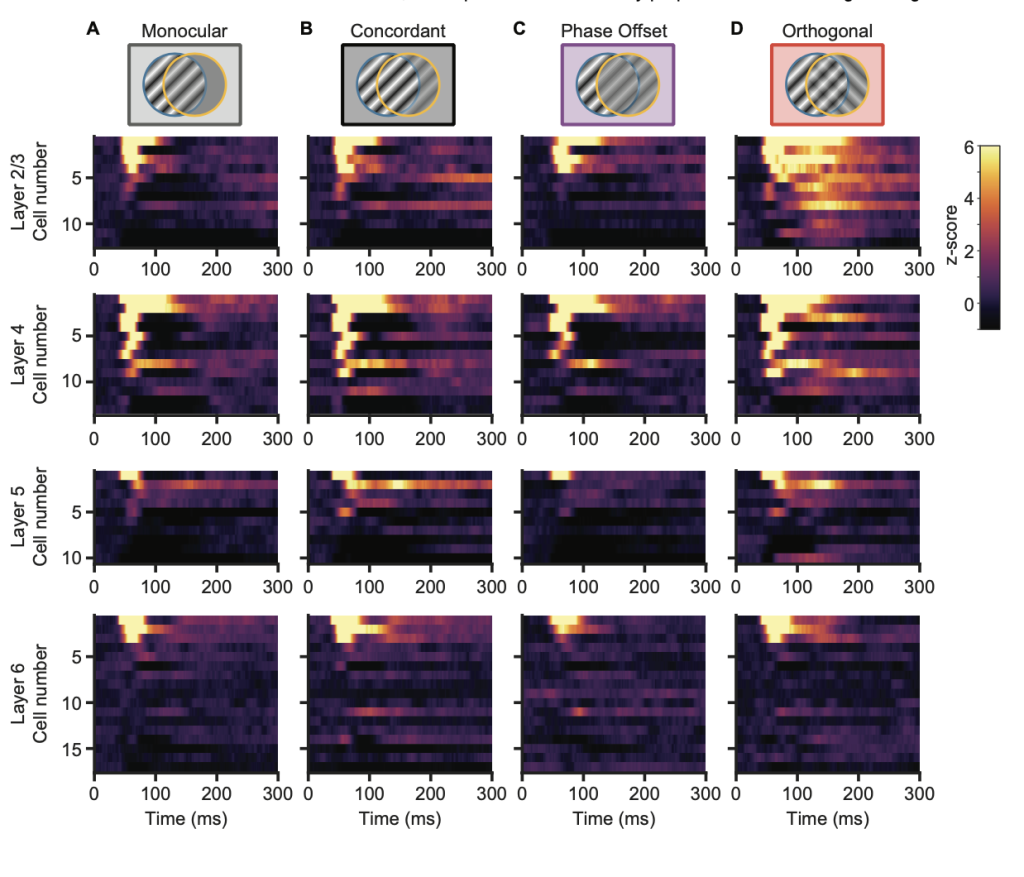
Figure 2. Fast-spiking single unit responses to different stimulus conditions. Preprint figure placed in the Public Domain.
Z-scored raster plots of regular-spiking (RS) single units for Monocular (A), Concordant (B), Phase Offset (C), and Orthogonal (D) stimuli. Units were assigned to L2/3, L4, L5, or L6 based on the location of the electrode contact with the maximal single unit waveform, then sorted based on their average activity between 40-80ms in the Monocular condition.
5. Disinhibition drives sustained activity under orthogonal conflict
Two-photon calcium imaging identified a disinhibitory circuit mechanism underlying the prolonged activation observed during orthogonal stimulation. Activity of somatostatin-positive (SOM⁺) interneurons, which normally suppress excitatory and PV⁺ cells, was strongly reduced during orthogonal conflict, leading to sustained excitation in both populations. This finding links the suppression of SOM⁺ activity to persistent cortical firing, providing a cellular explanation for how interocular orientation conflict can maintain extended activity associated with binocular rivalry.
What I like about the preprint?
To me, what is most compelling about these findings is how a single cortical network, mouse V1, flexibly reorganizes its activity depending on the nature of binocular conflict. The same neurons that typically fuse signals from both eyes can, under conflicting conditions, shift into distinct response modes either suppressing early input or sustaining prolonged activation. This adaptability reveals a remarkable computational versatility within the primary sensory cortex, showing that early visual areas are not merely feedforward filters but dynamic processors sensitive to context and stimulus congruence.
Equally striking is the ability to trace these divergent network states to specific interneuron mechanisms, particularly the unexpected disinhibition of SOM⁺ interneurons during orientation conflict. Even minor binocular mismatches can reconfigure inhibitory dynamics, revealing the fine-tuned adaptability of cortical networks. It is fascinating that such a localized circuit adjustment could underlie processes like binocular rivalry linking the dynamics of single interneuron classes to the broader question of how the brain resolves disagreement between the eyes.
Questions to the authors:
- Is interocular suppression in mouse V1 sufficient to drive perceptual exclusion?
- What are the developmental and plasticity mechanisms shaping binocular conflict responses?
- Are there distinct behavioral consequences associated with each form of binocular conflict in mice?
Future directions:
While the preprint in itself covers a wide range of questions regarding biocularity, it would be interesting to see what is the role of VIP+ interneurons in interocular conflict resolution? Given that vasoactive intestinal peptide-expressing (VIP+) interneurons disinhibit SOM+ cells, they are strong candidates for top-down or lateral modulation in binocular conflict. Their role remains unexplored in this context.
doi: https://doi.org/10.1242/prelights.41880
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the animal behavior and cognition category:
Cannibalism as a mechanism to offset reproductive costs in three-spined sticklebacks
Tina Nguyen
Morphological variations in external genitalia do not explain the interspecific reproductive isolation in Nasonia species complex (Hymenoptera: Pteromalidae)
Stefan Friedrich Wirth
Trade-offs between surviving and thriving: A careful balance of physiological limitations and reproductive effort under thermal stress
Tshepiso Majelantle
Also in the neuroscience category:
PPARδ activation in microglia drives a transcriptional response that primes phagocytic function while countering inflammatory activation
Isabel Paine
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Self-renewal of neuronal mitochondria through asymmetric division
Lorena Olifiers
preLists in the animal behavior and cognition category:
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Bats
A list of preprints dealing with the ecology, evolution and behavior of bats
| List by | Baheerathan Murugavel |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Also in the neuroscience category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |











 (1 votes)
(1 votes)