A major histocompatibility complex (MHC) class II molecule that binds the same viral pathogen peptide with both nonamer and decamer core sequences for presentation to T cells
Posted on: 5 November 2025 , updated on: 6 November 2025
Preprint posted on 27 October 2025
Do you love fashion shows? Well, chicken’s MHC class II is an immune fashion icon that presents the same viral peptide to T cells in both a 9- and 10-amino acid outfit.
Selected by Mitchell SarmieCategories: immunology, microbiology
Why I Picked This Preprint
As a graduate student in Infectious Diseases and One Health, I chose to highlight the novel findings in this preprint on structural and molecular host-pathogen interactions between chickens and Marek’s Disease Virus (MDV), which contribute to chickens’ resistance to the disease. The impact of this work extends beyound chicken’s immunity as it advances our broader knowledge of virulence and resistance, and supports the One Health approach by providing models for similar viral diseases affecting other species.
Background
Infectious diseases, such as Marek Disease Virus (MDV), Influenza, Newcastle Disease Virus (NDV), are a primary cause of economic loss in poultry farming1. As can be observed in all viral hosts, the chicken immune system has been evolving new structural and molecular mechanisms to resist these diseases23. Intriguingly, chicken Major Histocompatibility Complex (MHC) polymorphism shows relatively strong associations with infectious diseases’ peptide fragments compared to mammals, which facilitate resistance to MDV4.
Key Findings
- Plasticity in Peptide Binding by Chicken MHC Class II (BL2*021:01)
Chickens have a special immune protein (BL2*021:01) that can present the same viral peptide to two distinct binding motifs on the MHC. As illustrated in preprint Figures 2 to 4, the MHC class II MDV-resistant B21 haplotype can present peptide pp38 through the nonamer core and a decamer core. Specifically, preprint Figure 3 clearly showed the structural comparison of chicken and human MHC class II complexes, revealing unique features in peptide presentation that may underlie enhanced MDV resistance in BL2*021:01.
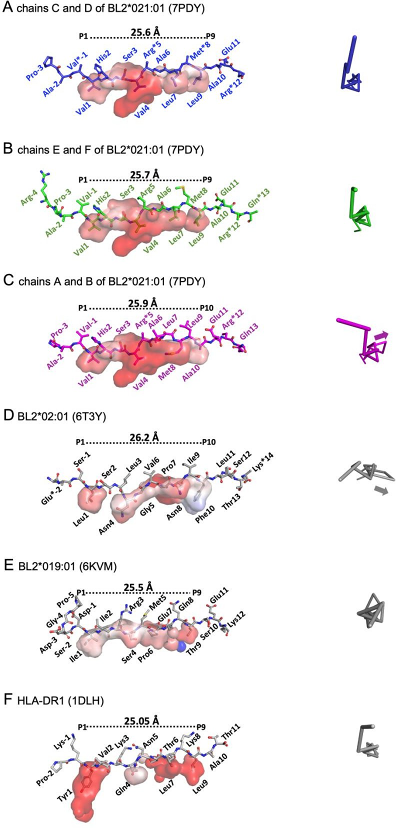
Figure 3: Representative configurations of peptide–MHC class II complexes from chickens (BL2*021:01, BL2*02:01, BL2*019:01) and humans (HLA-DR1), emphasising the associated antigenic peptides. The surface groove and the distance between anchor residues (P1 to P9 or P10) are displayed for each complex. Varied orientations and peptide interactions across MHC molecules demonstrate disparities in antigen presentation relevant to resistance to Marek’s Disease Virus (MDV).
This plasticity is unusual during antigenic peptide presentation: usually there are only weak MHC-peptide bonds between mammals and infectious diseases. The ability of BL2*021:01 to bind one peptide to two separate conformations portrays a clear picture of the diversity of molecular surface markers presented to CD4+ T cells in chickens. During clonal expansion of T cells, this plasticity may enhance the number of clones activated by a single peptide to drive resistance. This is also a possible explanation for the high frequency of the B21 haplotype among commercial chickens, as they can survive the wrath of infectious diseases, specifically MDV, in poultries and reach the market.
- Structural Basis for Divergent Peptide Binding Modes
The structural comparison of the BL2*021:01 molecule with the B2 haplotype decamer core motif that is associated with resistance to MDV infection in chickens (BLB*02:01 B2), susceptible BL2*19:01 B19, and human HLA-DR1 counterparts reveals distinct differences within the peptide-binding groove that govern peptide interaction and disease outcome. Specifically, variations in key amino acid residues within the BLB chain modify the local groove architecture, promoting a unique decameric (10-amino-acid core) peptide binding mode. This conformation arises from a subtle “crinkle” or distortion in the peptide backbone at defined positions, dictated by polymorphic contacts between the peptide and the MHC binding pockets. These structural adjustments describe molecular determinants underlying resistance or susceptibility phenotypes.
The crystallographic analysis confirms that this decameric core configuration is not an artefact of crystal packing or monomer associations, which is an important point for understanding the underlying molecular mechanism of chicken resistance to MDV. To support this even more, a thorough analysis of intermolecular interactions among various crystal forms (illustrated in preprint Figures 7 and 8) indicate that the identified plasticity binding pattern observed in BL2*021:01 represents a true biological characteristic, emphasising the inherent flexibility and adaptability of the class II peptide-binding groove to accommodate diverse peptide conformations.
Key Contributions
- Chicken MHC decameric core sequence: This preprint has for the first time revealed that chicken MHC II simultaneously binds MDV peptide to a 10-amino-acid core and the classical 9 amino-acid core that resist the MDV by enhancing T lymphocytes response during host-pathogen interaction.
- These findings present a novel MHC architecture in chickens that could inform future research on other infectious diseases like avain influenza, infectious bronchitis virus, Newcastle Disease Virus, etc, antigenic presentation within chickens.
Questions and Future Directions
While this preprint presents novel knowledge in MHC biology, there are further questions that still need to be answered to direct future research. Just a few of these questions are:
- Can the plasticity property of the B21 haplotype be transferred or induced in susceptible chicken cell lines through manipulation or other cellular and molecular means?
- During the immune response, what is the functional significance of presenting the same peptide to the nonamer core and a decamer core for T cell clonal expansion and activation?
- Is this Chicken’s MHC plasticity only specific to MDV? If not, what other infectious dieases undergo this process during peptide presentation and why is this not driving resistance?
Bibliography
- Muñoz-Gómez, V. et al. Economic impact of chicken diseases and other causes of morbidity or mortality in backyard farms in low-income and middle-income countries: a systematic review and meta-analysis. BMC Vet Res 21, 151 (2025).
- Golpasand, S., Ghovvati, S. & Pezeshkian, Z. Exploring the molecular and biological mechanisms of host response in chickens infected with highly pathogenic avian influenza virus (H5N1): An integrative transcriptomic analysis. PLoS One 20, e0332689 (2025).
- Berihulay, H. et al. Exploring the genetic basis of Newcastle disease virus in chickens: a comprehensive review. Front. Immunol. 16, 1614794 (2025).
- Kaufman, J. Generalists and Specialists: A New View of How MHC Class I Molecules Fight Infectious Pathogens. Trends in Immunology 39, 367–379 (2018).
doi: https://doi.org/10.1242/prelights.42027
Read preprintThe study focused on the novel finding of an MHC class II molecule (BL2*021:01) in chickens that can bind the same viral peptide in two different core conformations. This plasticity is proposed as a key determinant of resistance to MDV seen in chickens with the B21 haplotype, common in commercial breeds. However, it does not provide experimental specifics about breed, housing, prior exposure, vaccination, or medication. The primary experimental system relies on cell lines, recombinant protein expression, and birds with the B21 haplotype. If you need more methodological or experimental details, please contact the authors. You can find their emails in the preprint.
D. Samuel Kollie, Jr.
Quite an interesting work. It is clear that the impact of this preprint extends beyond chicken immunity to MDV. The principles it presents could be used to study and understand resistance to other animal diseases, which is critical for preventing the economic losses caused by livestock infections worldwide and for reducing the transmission of zoonotic diseases.
My questions are:
1. Were the viral peptide conformations (binding orientations) influenced by any experimental factors, or were they only observed and studied?
2. Can the genes that encode B21 be altered genetically to induce resistance to MDV or potentially applied to other animal models?
Have your say
Sign up to customise the site to your preferences and to receive alerts
Register hereAlso in the immunology category:
A Novel Chimeric Antigen Receptor (CAR) - Strategy to Target EGFRVIII-Mutated Glioblastoma Cells via Macrophages
Dina Kabbara
Loss of MGST1 during fibroblast differentiation enhances vulnerability to oxidative stress in human heart failure
Jeny Jose
Scalable transcription factor mapping uncovers the regulatory dynamics of natural and synthetic transcription factors in human T cell states
Inês Caiado
Also in the microbiology category:
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Microbial Feast or Famine: dietary carbohydrate composition and gut microbiota metabolic function
Jasmine Talevi
Citrobacter rodentium infection activates colonic lamina propria group 2 innate lymphoid cells
André Luiz Amorim Costa, Marcus Oliveira
preLists in the immunology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |
Also in the microbiology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |











 (No Ratings Yet)
(No Ratings Yet)
4 months
Yahaya Sakwa
This is great,
I have a few discussion points.
I went through the publication both yours and the authors, hoping to find details on the breeds of chicken, location, what prior exposure they have had, vaccination and medications, are they genetically modified… etc…