Conserved cardiolipin-mitochondrial ADP/ATP carrier interactions assume distinct structural and functional roles that are clinically relevant
Posted on: 14 July 2023 , updated on: 19 June 2024
Preprint posted on 6 May 2023
Article now published in at https://www.embopress.org/doi/full/10.1038/s44318-024-00132-2
Reduced power supply without cardiolipin: mechanisms behind a mitochondrial myopathy mutation in the ADP/ATP carrier revealed
Selected by Barbora KnotkovaCategories: biochemistry
Background:
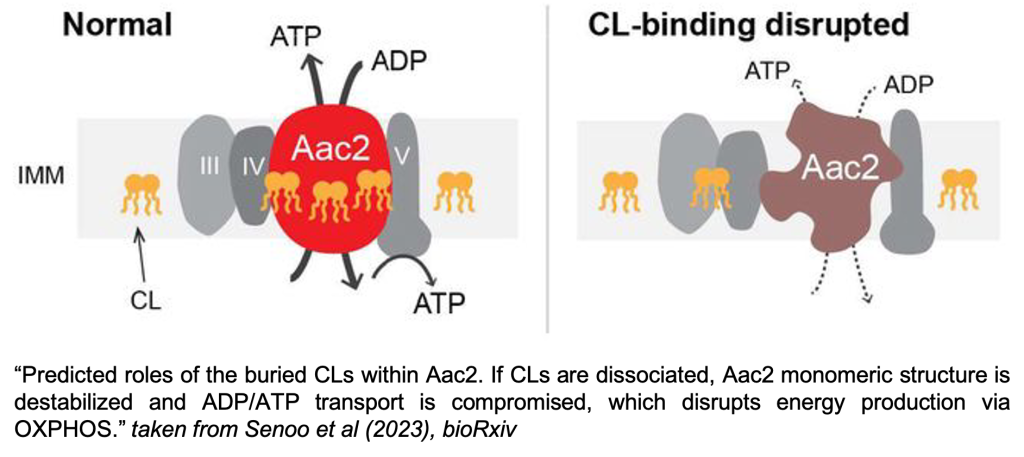
Most energy used by our cells is produced in mitochondria in the form of ATP. Therefore, mitochondria are full of proteins devoted to this task. Alongside components of the respiratory chain complexes, another protein supporting energy production in mitochondria is the ADP/ATP carrier (Aac in yeast; ANT in humans), a member of the SLC25 solute carrier family. It sits in the inner mitochondrial membrane and imports ADP into the mitochondrial matrix for use by the ATP synthetase, while at the same time exporting the generated ATP for use by the rest of the cell. Available structures of the ADP/ATP carrier show up to three cardiolipin molecules bound to distinct pockets of the protein [1-3]. Cardiolipin is a lipid found predominantly in the inner mitochondrial membrane, where it is known to stabilise several prominent proteins and protein complexes [4, 5]. Even though there were hints that the cardiolipins may support the stability and function of the ADP/ATP carrier[6-9], this preprint is the first to provide experimental verification for this and to link this lipid-protein interaction to a clinically relevant mutation.
Model generation:
To investigate how cardiolipin (CL) binding influences the stability and function of the ADP/ATP carrier, mutations were introduced in the previously identified CL binding pockets 1 to 3 of the ADP/ATP carrier in yeast: Aac2. Residues in the binding pocket’s vicinity were mutated to negatively charged amino acids in order to disrupt CL binding by electrostatic repulsion of the CL headgroups. These mutant yeast Aac2 variants did not display defects in expression level or topology. Native mass spectrometry could confirm that three CL-specific lipid binding pockets exist in Aac2, and that the designed mutations indeed weaken binding of CL to the Aac2 carrier.
Key findings:
1) CL binding stabilises Aac2’s monomeric structure
As already shown in the authors’ previous study [9], removing CL from the cell leads to the destabilisation of the 140kDa Aac2 monomer into a smear on a blue native-PAGE. The mutations in CL binding pockets generated in the present study destabilise the monomeric structure in the same way, even though CL is present in the cell. This clearly points to a direct role of CL in stabilizing Aac2’s structure via its binding. It should be mentioned, however, that the observed destabilisation only occurs after detergent-mediated extraction from the cell, as conformation-stabilising inhibitors of Aac2 can rescue the destabilising effect when added to the cells.
2) Bound CL is important for Aac2 transport activity, and exerts its strongest effect through binding pocket 2
Native mass spectrometry data revealed that one of the binding pockets has lower affinity towards CL than the other two. The elevated importance of one of the pockets also became apparent in functional studies. Pocket 2 mutants displayed the biggest decrease in ADP/ATP transport activity measured on isolated mitochondria, followed by pocket 3 and 1 mutants, respectively. These findings were further supported by oxygen consumption measurements, which detected lower respiration activity due to reduced ADP import in isolated mitochondria from pocket 2 and 3 mutants compared to WT mitochondria.
3) CL-Aac2 interaction supports respiratory complexes
Cox1-3 are subunits of the respiratory chain complex encoded in mitochondrial DNA. It has previously been shown that ADP/ATP transport modulates the expression of these subunits. Mutants with the most reduced Aac2 activity also showed the lowest expression of Cox1-3. Furthermore, several mutants could not maintain a stable interaction between Aac2 and the respiratory supercomplex seen in WT. Again, the pocket 2 mutants were most affected.
4) An existing pathogenic mutation leads to the loss of CL association with human Ant1 and has an effect on the transporter’s structure and function
Having established that CL binding is important for the structure and function of yeast Aac2, the authors examined an as of yet uncharacterised mutation in the human orthologue ANT1: Leucine 141, localised to pocket 2, replaced by phenylalanine (L141F). The patient harbouring this recessive mutation suffers from mitochondrial myopathy [10].
The pathogenic mutation was introduced in ANT1 in human cells as well as in Aac2 in yeast cells and a very similar phenotype to the previously characterised CL-binding mutants could be observed. This included reduced CL binding in yeast; and reduced monomeric stability, ADP/ATP exchange capacity and oxygen consumption rate in human cells.
Molecular dynamics simulations were performed to investigate CL interactions with ANT1. CL was less likely to associate with pocket 2 of the mutant ANT1 than the WT ANT1 when the pocket was left unoccupied at the start of the simulation. In addition to potentially providing a steric hinderance, the L141F mutation also led to higher fluctuations in other residues of pocket 2 in the simulation, destabilising the CL binding environment. Another computational method was employed to calculate that the binding free energy of CL is around 12 kcal/mol lower for the WT than for the mutant ANT1.
What I like about the preprint:
I am interested in inner mitochondrial membrane proteins and lipids, which is why I selected this preprint. There is an increasing number of studies emerging, including structural data, which show that cardiolipin is important for the stability and function of mitochondrial membrane proteins. I liked that the authors tried to answer the question of how exactly cardiolipin may be important. In this preprint, Senoo and co-authors could directly attribute the defects seen in their mutants to the loss of a very specific lipid-protein interaction. Moreover, they could show that the same phenotype can be caused by a pathological mutation in humans, providing strong evidence for the physiological significance of these specific lipid-protein interactions.
Questions for the authors:
1) Do you have any ideas on how CL is involved in mechanisms of ADT/ATP transport? For example, how CL binding and unbinding may contribute to conformational changes between the c-state and the m-state? What kind of methods could one employ to dig even deeper into these kind of questions?
2) As the binding between the ADP/ATP carrier and cardiolipin is based on electrostatic interactions, do you think that other negatively charged lipids may be able to compensate for the lack of CL, if present in sufficient concentrations?
3) What did you enjoy the most while working on this study? 🙂
References:
1. Pebay-Peyroula, E., et al., Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature, 2003. 426(6962): p. 39-44.
2. Nury, H., et al., Structural basis for lipid-mediated interactions between mitochondrial ADP/ATP carrier monomers. FEBS Lett, 2005. 579(27): p. 6031-6.
3. Ruprecht, J.J., et al., Structures of yeast mitochondrial ADP/ATP carriers support a domain-based alternating-access transport mechanism. Proc Natl Acad Sci U S A, 2014. 111(4): p. E426-34.
4. Acoba, M.G., N. Senoo, and S.M. Claypool, Phospholipid ebb and flow makes mitochondria go. J Cell Biol, 2020. 219(8).
5. Paradies, G., et al., Role of Cardiolipin in Mitochondrial Function and Dynamics in Health and Disease: Molecular and Pharmacological Aspects. Cells, 2019. 8(7).
6. Crichton, P.G., et al., Trends in thermostability provide information on the nature of substrate, inhibitor, and lipid interactions with mitochondrial carriers. J Biol Chem, 2015. 290(13): p. 8206-17.
7. Yi, Q., et al., The effects of cardiolipin on the structural dynamics of the mitochondrial ADP/ATP carrier in its cytosol-open state. J Lipid Res, 2022. 63(6): p. 100227.
8. Montalvo-Acosta, J.J., et al., Structure, substrate binding, and symmetry of the mitochondrial ADP/ATP carrier in its matrix-open state. Biophys J, 2021. 120(23): p. 5187-5195.
9. Senoo, N., et al., Cardiolipin, conformation, and respiratory complex-dependent oligomerization of the major mitochondrial ADP/ATP carrier in yeast. Sci Adv, 2020. 6(35): p. eabb0780.
10. Tosserams, A., et al., Two new cases of mitochondrial myopathy with exercise intolerance, hyperlactatemia and cardiomyopathy, caused by recessive SLC25A4 mutations. Mitochondrion, 2018. 39: p. 26-29.
doi: https://doi.org/10.1242/prelights.35101
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the biochemistry category:
Active flows drive clustering and sorting of membrane components with differential affinity to dynamic actin cytoskeleton
Teodora Piskova
Snake venom metalloproteinases are predominantly responsible for the cytotoxic effects of certain African viper venoms
Daniel Osorno Valencia
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
preLists in the biochemistry category:
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
Peer Review in Biomedical Sciences
Communication of scientific knowledge has changed dramatically in recent decades and the public perception of scientific discoveries depends on the peer review process of articles published in scientific journals. Preprints are key vehicles for the dissemination of scientific discoveries, but they are still not properly recognized by the scientific community since peer review is very limited. On the other hand, peer review is very heterogeneous and a fundamental aspect to improve it is to train young scientists on how to think critically and how to evaluate scientific knowledge in a professional way. Thus, this course aims to: i) train students on how to perform peer review of scientific manuscripts in a professional manner; ii) develop students' critical thinking; iii) contribute to the appreciation of preprints as important vehicles for the dissemination of scientific knowledge without restrictions; iv) contribute to the development of students' curricula, as their opinions will be published and indexed on the preLights platform. The evaluations will be based on qualitative analyses of the oral presentations of preprints in the field of biomedical sciences deposited in the bioRxiv server, of the critical reports written by the students, as well as of the participation of the students during the preprints discussions.
| List by | Marcus Oliveira et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
20th “Genetics Workshops in Hungary”, Szeged (25th, September)
In this annual conference, Hungarian geneticists, biochemists and biotechnologists presented their works. Link: http://group.szbk.u-szeged.hu/minikonf/archive/prg2021.pdf
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |











 (1 votes)
(1 votes)