Hydrophobic-cationic peptides enhance RNA polymerase ribozyme activity by accretion
Posted on: 23 April 2021
Preprint posted on 23 February 2021
Article now published in Nature Communications at http://dx.doi.org/10.1038/s41467-022-30590-3
Accretion in the prebiotic world: simple peptides might pave the way to understanding the history of biological life
Selected by Utrecht Protein Folding and AssemblyCategories: biochemistry
Written by: Julia van den Boogert, Isabel van Rongen, Emile van Weert & Olivier Zadelhoff
Background
The origin of life is thought to have arisen from an RNA world in which ribozymes catalysed biochemical reactions and RNA stored genetic information. However, the emergence of RNA-based life required compartmentalization, or otherwise RNA replication systems would be overrun and genetic selection could not have occurred. Compartmentalization may have been achieved by accretion of peptides and RNA. The term accretion implies that biopolymers gradually accumulate into macromolecular aggregates stabilized by intermolecular forces. Accretion of peptides and RNA could lead to an increased local concentration and a basic form of compartmentalization, which may have played a role in the origin of life. However, the impact of accretion on ribozymes is largely unknown.
Examining the accretion hypothesis, Li et al. discover the RNA binding peptide P43 (AKKVWIIMGGS), which is able to accrete multiple ribozyme species and regulate their activity.
Key findings
The authors obtain P43 by performing a randomized peptide phage display, selecting for peptides that bind and regulate RNA polymerase ribozyme (RPR). P43 is a cationic-hydrophobic peptide which forms insoluble aggregates that can accrete RNA and ribozymes on its surface. The phage display reveals that P43 inhibits RPR activity. By assessing the inhibiting potential of truncated P43 variants, the authors find that not only hydrophobic but also cationic residues are crucial in forming P43 aggregates and in accretion, indicating a role for electrostatic interactions between cationic residues and anionic RNA. This differs from a previously studied, purely cationic, non-aggregating (Lys)10 prebiotic peptide (Tagami et al., 2017).
P43 shows preferential binding for longer RNA strands, hence acting as a suitable platform for the formation of longer biopolymers. By showing that the shorter and simpler peptide (Lys2)(Val)6 is also able to inhibit RPR activity, the authors prove that the P43 inhibitory efficacy is a general feature of cationic-hydrophobic peptides. This simplification is a proof of concept, making the accretion model even more plausible in evolutionary terms.
The prebiotic hypothesis posits that salt concentrations frequently fluctuated. Because of this, the authors are curious about the influence of salt concentration on the P43-RPR interaction. Low salt allows P43 aggregates to inhibit RPR, likely because the electrostatic interactions with RNA are so tight that they distort the ribozyme structure. At high salt concentrations, loosened interactions between accreted RNA and P43 enhance RPR activity. Next, the authors demonstrate that P43 captures inactive ribozymes at low salt, and reactivates them by subsequent resuspension in high salt concentrations.
These findings suggest a potential role for freeze/thaw cycles in the prebiotic world. Diurnal cycling of wet and dry periods brought about the occurrence of three conditions: dry, wet and condensed. The dry phases would have favored biopolymer formation due to dehydration; subsequent wet conditions the accretion of biomaterials through the influx of water. Entering condensed conditions through evaporation of water, the enhancement of ribozyme activity by peptide aggregates would have occurred, as salt concentrations increased (Figure 6). In short, it is likely that freeze/thaw cycles in combination with cationic/hydrophobic peptides like P43 enabled RNA polymerization without the use of complicated biochemical pathways.
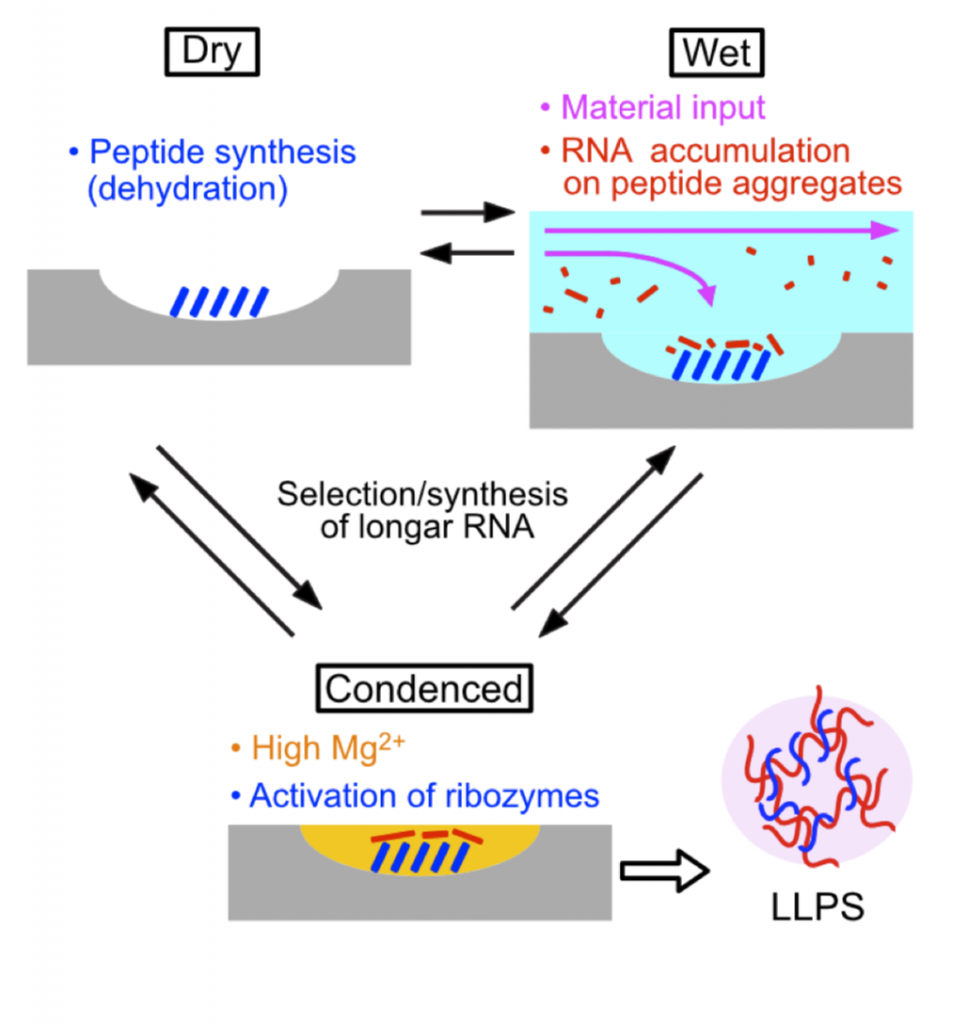
The authors of this preprint advocate the importance of accretion in the formation of prebiotic peptides. As accretion leads to a gradual increase in RNA and peptide concentration, it might have preceded the formation of liquid-like compartments. Ultimately, compartmentalization through accretion protects from environmental stress factors, thereby enabling the retainment of novel functions. Consequently, bulk P43-like peptide synthesis would pose evolutionary advantages even before the emergence of cell-like life.
What we like about this preprint
Compartmentalization is crucial for the origin of life, however there is no consensus as to how this was attained. It is interesting to see how the authors describe and demonstrate that compartmentalization can be achieved through accretion. We like the simplicity of the accretion model as one of the first steps towards the immensely complex emergence of life. We believe that this paper is an extension to our knowledge on the origin of life and can perhaps grant insights in other fields of biochemical research.
References
- Attwater, J., Wochner, A., & Holliger, P. (2013). In-ice evolution of RNA polymerase ribozyme activity. Nature chemistry, 5(12), 1011–1018.
- Tagami, S., Attwater, J., & Holliger, P. (2017). Simple peptides derived from the ribosomal core potentiate RNA polymerase ribozyme function. Nature chemistry, 9(4), 325–332.2
doi: Pending
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the biochemistry category:
Active flows drive clustering and sorting of membrane components with differential affinity to dynamic actin cytoskeleton
Teodora Piskova
Snake venom metalloproteinases are predominantly responsible for the cytotoxic effects of certain African viper venoms
Daniel Osorno Valencia
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
preLists in the biochemistry category:
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
Peer Review in Biomedical Sciences
Communication of scientific knowledge has changed dramatically in recent decades and the public perception of scientific discoveries depends on the peer review process of articles published in scientific journals. Preprints are key vehicles for the dissemination of scientific discoveries, but they are still not properly recognized by the scientific community since peer review is very limited. On the other hand, peer review is very heterogeneous and a fundamental aspect to improve it is to train young scientists on how to think critically and how to evaluate scientific knowledge in a professional way. Thus, this course aims to: i) train students on how to perform peer review of scientific manuscripts in a professional manner; ii) develop students' critical thinking; iii) contribute to the appreciation of preprints as important vehicles for the dissemination of scientific knowledge without restrictions; iv) contribute to the development of students' curricula, as their opinions will be published and indexed on the preLights platform. The evaluations will be based on qualitative analyses of the oral presentations of preprints in the field of biomedical sciences deposited in the bioRxiv server, of the critical reports written by the students, as well as of the participation of the students during the preprints discussions.
| List by | Marcus Oliveira et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
20th “Genetics Workshops in Hungary”, Szeged (25th, September)
In this annual conference, Hungarian geneticists, biochemists and biotechnologists presented their works. Link: http://group.szbk.u-szeged.hu/minikonf/archive/prg2021.pdf
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |











 (2 votes)
(2 votes)