Microglia become hypofunctional and release metalloproteases and tau seeds after phagocytosing live neurons with P301S tau aggregates
Posted on: 11 March 2021
Preprint posted on 26 February 2021
The bitter taste of tau: how microglia “eating” neurons with tau aggregates may contribute to disease pathology and spreading in tauopathies
Selected by Kristina KuhbandnerCategories: neuroscience
Background
Aggregation of the protein tau is observed in several human neurodegenerative diseases including Alzheimer’s disease, frontotemporal dementia (FTD) and Pick’s disease. These tauopathies are characterized by neuronal cell death associated with intracellular tau aggregates (1). The exact mechanism of cell death and spreading of tau in the central nervous system (CNS) remains unclear, but there is evidence that microglia, the resident immune cells in the brain, are involved (2).
Amongst others, activated microglia help to remove damaged or dying cells in the CNS by engulfing them in a process known as phagocytosis. This is triggered by the exposure of “eat-me” signals like phosphatidylserine (PtdSer) on the damaged cells (3). Previously, the Spillantini lab investigated phagocytosis in co-cultures of microglia with neurons isolated from P301S tau transgenic mice. Due to the expression of mutant tau protein, these mice develop neuronal tau aggregates starting at the age of 3 months (4). The respective study revealed that living neurons with tau inclusions exhibit abnormally high amounts of PtdSer marking them for phagocytosis by microglia (5). Using the same model, in this pre-print Brelstaff et al. analyzed microglia that phagocytosed tau-containing neurons in more detail.
Key results
- Microglia phagocytosing neurons with tau aggregates release tau
First, microglial cells from C57BL/6 wildtype mice were cultured with neurons isolated from 5-month-old P301S tau transgenic mice (P301S neurons). After several days, a significant amount of tau was detected in the supernatant of these co-cultures. Blocking phagocytosis by masking PtdSer markedly reduced the loss of neurons and the presence of tau in the medium indicating that phagocytosing microglia were the source of tau. Remarkably, microglia continued to release tau when re-isolated from the neuronal co-cultures.
- Released tau is insoluble and can trigger seeding of tau aggregates
Then, they further characterized tau detected in co-culture medium. Similar to tau in P301S neurons, tau in the supernatant was present in the form of insoluble aggregates. Of note, the isolated species were also able to induce aggregation in a tau seeding assay.
- After phagocytosing tau aggregate-containing neurons microglia become hypophagocytic and adopt a senescence-like state
To determine the phagocytic capacity of microglia after ingesting P301S neurons, microglia were re-isolated and cultured again in the presence of fresh P301S neurons. In this setting, no significant loss of tau aggregate-containing neurons was observed indicating a hypophagocytic phenotype which renders microglia unable to engulf other neurons with tau aggregates. Additionally, these microglia showed an increase in acidic β-galactosidase activity, a marker for cellular senescence.
- Hypophagocytic microglia release a specific protein profile enriched in MMP3
Next, a proteome array was performed on co-culture medium to identify proteins associated with the observed alterations in microglia functionality. While microglial monocultures released proteins such as CCL2, CCL6 and VEGF, addition of LPS induced a more inflammatory phenotype including TNFα expression. In contrast, no pro-inflammatory cytokines were detected in the medium of untreated P301S neuron-microglia co-cultures. Instead, several senescence-associated factors were secreted with matrixmetalloprotease 3 (MMP3) being the topmost upregulated. Consequently, the authors analyzed MMP3 expression in mouse and human brain tissue associated with tauopathies. Its active form was not only found to be upregulated in aged P301S mice but also in the brain of patients with different neurodegenerative diseases such as FTD and Pick’s disease.
- MMP3 expression is regulated by the NFκB pathway
Several of the upregulated proteins are controlled by the NFκB pathway. To test whether this also applies to MMP3, microglia were pre-treated with an NFκB pathway inhibitor; this resulted in a reduction of MMP3 in the culture medium. Interestingly, inhibition of phagocytosis did not decrease MMP3 production indicating that PtdSer-signalling is not involved in MMP3 activation.
Graphical summary
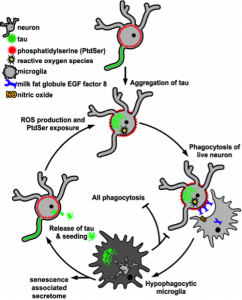
Figure 1. Potential role of microglia in a vicious cycle after phagocytosing neurons with tau aggregates.
Microglia that have phagocytosed tau aggregate-containing neurons acquire a hypophagocytic, senescence-like state and might enhance tau pathology by spreading tau aggregates (taken from Brelstaff et al., 2020, Fig. 5, made available under a CC-BY-NC 4.0 International license).
Why I chose this preprint
There are several reasons why I highlighted this pre-print. First, in combination with previous findings, this study contributes to the understanding of cell death mechanisms and the spreading of tau aggregates in tauopathies. Moreover, it provides further evidence that preventing neurodegeneration and phagocytosis is a promising treatment strategy for tauopathies. Second, one of my research projects deals with phagocytosis of PtdSer-expressing living motor neurons in the context of amyotrophic lateral sclerosis, another neurodegenerative disease with characteristic protein aggregations. Thus, I am curious whether the reported results are also applicable to other tau-independent neurodegenerative disorders. Third, March 8th is celebrated as International Women’s Day. In most professions, including academia and science, women are still underrepresented at higher career stages. Early data suggest that the COVID-19 pandemic especially affected the work of female scientists and could widen the gender gap (6). Here, I like to emphasize that this study is led by Maria Gracia Spillantini, a renowned female neuroscience expert who is probably best known for identifying alpha-synuclein as disease-associated aggregation-prone protein in Parkinson’s disease. With her remarkable achievements and her extremely successful academic career, she is an encouraging role model inspiring many young scientists.
Questions to the authors
- In your co-culture setting naïve microglia from C57BL/6 mice were used. Do you expect microglia isolated from aged P301S mice to behave different? Are there indications that these have already adopted a senescent-like state as you observed for naïve microglia after culture with P301S neurons?
- The findings of this study are mainly based on the analysis of culture media. Do you also plan to further characterize senescent microglia, for example by RNAseq?
- Phagocytosis of living neurons is a phenomenon observed in various neurodegenerative diseases (3). Can you speculate to what extend your findings might also be transferable to other diseases involving protein aggregates such as Parkinson’s disease or amyotrophic lateral sclerosis?
- Spillantini, you have started your enormously successful career more than 30 years ago. Do you think that today achieving a meaningful position in academia as female scientist is easier and if so, why? What needs to be done in the future in this regard?
Literature
- Spillantini MG, Goedert M. Tau pathology and neurodegeneration. Lancet Neurol. 2013 Jun 1;12(6):609–22.
- Perea JR, Llorens-Martín M, Ávila J, Bolós M. The Role of Microglia in the Spread of Tau: Relevance for Tauopathies. Front Cell Neurosci [Internet]. 2018 [cited 2021 Mar 6];12. Available from: https://www.frontiersin.org/articles/10.3389/fncel.2018.00172/full
- Brown GC, Neher JJ. Microglial phagocytosis of live neurons. Nat Rev Neurosci. 2014 Apr;15(4):209–16.
- Allen B, Ingram E, Takao M, Smith MJ, Jakes R, Virdee K, et al. Abundant Tau Filaments and Nonapoptotic Neurodegeneration in Transgenic Mice Expressing Human P301S Tau Protein. J Neurosci. 2002 Nov 1;22(21):9340–51.
- Brelstaff J, Tolkovsky AM, Ghetti B, Goedert M, Spillantini MG. Living Neurons with Tau Filaments Aberrantly Expose Phosphatidylserine and Are Phagocytosed by Microglia. Cell Rep. 2018 Aug 21;24(8):1939-1948.e4.
- Woitowich NC, Jain S, Arora VM, Joffe H. COVID-19 Threatens Progress Toward Gender Equity Within Academic Medicine. Acad Med J Assoc Am Med Coll. 2020 Sep 29;
doi: https://doi.org/10.1242/prelights.27633
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the neuroscience category:
Electrophysiological correlates of conscious experiences during sleep: Lucid dreams, sleep paralysis, out-of-body experiences, and false awakenings
uMontreal Neuro preLighters et al.
PPARδ activation in microglia drives a transcriptional response that primes phagocytic function while countering inflammatory activation
Isabel Paine
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
preLists in the neuroscience category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |











 (2 votes)
(2 votes)