SQ3370, the first clinical click chemistry-activated cancer therapeutic, shows safety in humans and translatability across species
Posted on: 6 May 2023 , updated on: 13 March 2024
Preprint posted on 29 March 2023
Click! In the body. @sangsri and colleagues (@DrTriHungNguyen, @jmejiaoneto) from @shasqi describe clinical click chemistry-activated cancer therapeutic platform. Preprint highlighted by @zhanghe_goh
Selected by Zhang-He GohCategories: pharmacology and toxicology
Background of the preprint
Doxorubicin is an anthracycline-based agent used in the treatment of many different cancers. Unfortunately, doxorubicin’s high toxicities—especially to the heart—limits the total dose that patients can take throughout their lifetime. This means that even where doxorubicin may previously have worked well in sending a patient’s cancer into remission, the patient and their healthcare professional team may have to consider alternative treatments should the cancer recur.
In this preprint, Srinivasan and co-workers describe a platform (named CAPAC*) for the specific activation of drugs in the desired microenvironment. Given that doxorubicin’s toxicity remains a problem in healthcare today, the authors decided to target doxorubicin using this platform.
Briefly, the CAPAC platform consists of two components, which we will call component α and β respectively (Figure 1). Component α is a tumour-localising agent that targets the tumour environment directly. Component β, called the protodrug, contains the drug payload. Component β is chemically modified: it exhibits neither activity nor toxicity alone, but once it attaches to component α, it releases the drug payload.
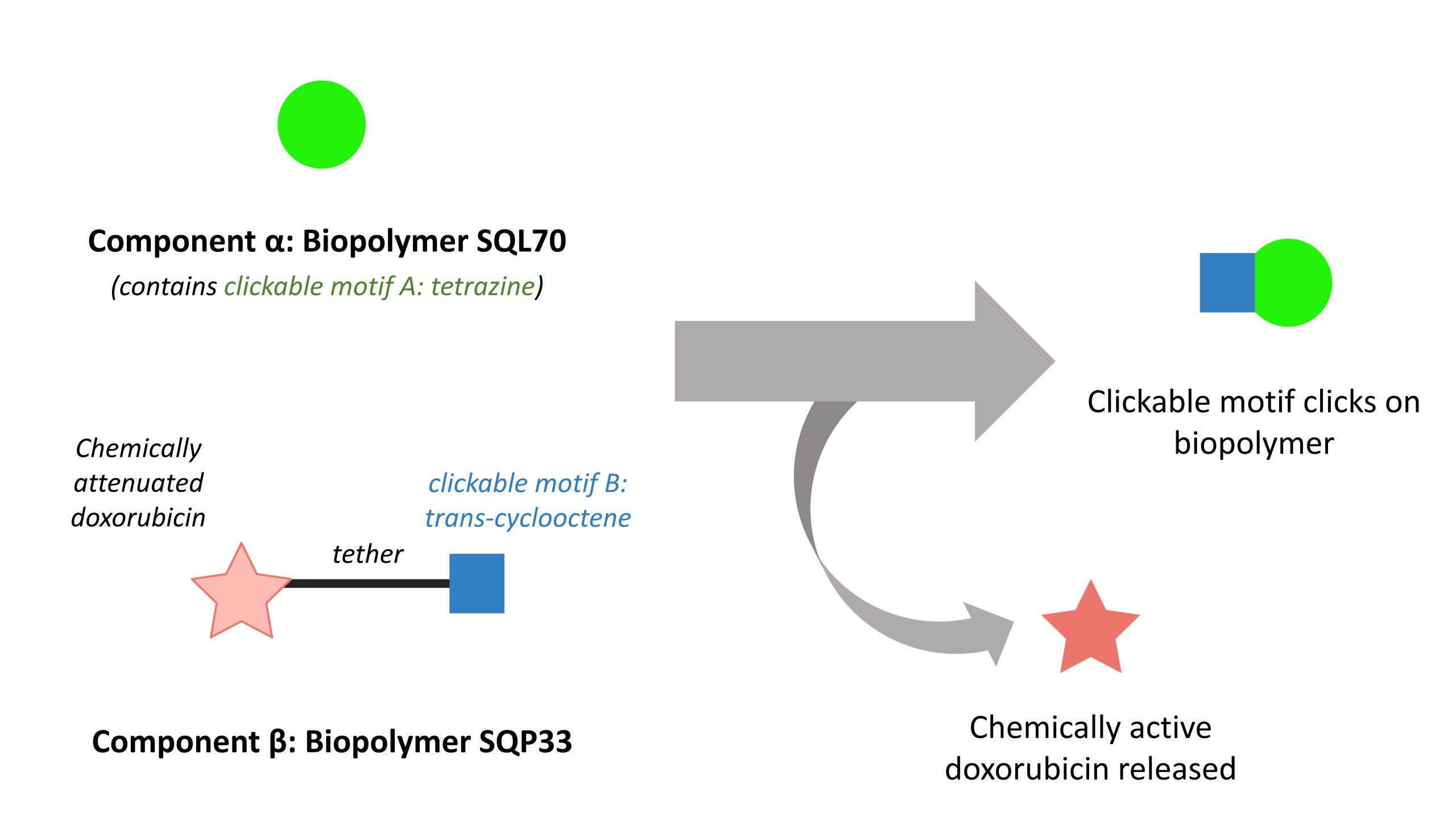
Figure 1. Principle underlying the CAPAC platform described by Srinivasan and co-workers.
Taken together, this means that the CAPAC platform undergoes a chemical reaction to release the payload within the desired biological environment. This process ensures that the payload is selectively released at the desired site, instead of being released in a non-selective manner throughout the body.
* CAPACTM is an abbreviation of Click Activated Prodrugs Against Cancer. It is trademarked by Shasqi, Inc.
Key findings of this preprint
In their preprint, Srinivasan and co-workers demonstrate that their CAPAC platform selectively and safely targets tumours and releases doxorubicin using SQ3370. SQ3370 consists of two components: component α is the SQL70 biopolymer, and component β is the doxorubicin-containing protodrug SQP33.
In SQ3370’s preclinical phase, Srinivasan and co-workers tested SQ3370 in mice and dogs. The authors conducted these experiments on four groups of mice: (A) a negative control with only saline, (B) a positive control with only Doxorubicin, (C) mice treated with SQ3370 at the tumour site, and (D) mice treated with SQ3370 at a site distal to the tumour. These experiments showed that localising SQ3370 in tumours (group C) generated better outcomes compared to SQ3370 being injected into the distal site (group D): the mice exhibited better antitumour responses and survived longer. Further mass-spectrometry experiments confirmed that doxorubicin was better released with SQ3370 treatment than with the appropriate controls.
The authors showed that SQ3370 was much less toxic than doxorubicin in dogs, requiring a dose equivalent to 9x that of doxorubicin to be fatal. Importantly, SQ3370 neither elicited cardiotoxicity nor a vesicant effect, further confirming that the release of doxorubicin was selective only at the intended sites. Subsequent toxicokinetic studies in dogs to study the release of doxorubicin from SQ3370 showed that the dogs had increased doxorubicin exposure when treated with SQ3370 compared to SQP33 alone. These studies confirm SQP33’s in vivo stability, broadly agreeing with the authors’ previous studies in rodents.
The preclinical studies of SQ3370 were followed by a phase I dose escalation clinical trial, for which the purpose was to determine the recommended phase II dose of SQ3370. In these dose escalation studies, the dose of the investigated drug is gradually increased until the maximum tolerated dose has been found. Interestingly, because the maximum tolerated dose had not been reached in this trial, the authors established it to be at 12x doxorubicin.
Srinivasan and co-workers found that the clinical studies of SQ3370 broadly agreed with their observations made in the preclinical studies. In fact, overall doxorubicin exposure increased in patients treated with SQ3370 compared to conventional doxorubicin, even as the maximum plasma concentration decreased. These results imply that higher exposure to doxorubicin may be possible with SQ3370 compared to conventional doxorubicin dosing. In these studies, the authors also found that the SQ3370 treatment induced cytotoxic lymphocyte activity in the patients’ tumours, an observation that agrees with previous preclinical studies in mice. This was particularly encouraging for two reasons. First, many patients had already undergone numerous cycles of anticancer treatment before enrolling in this trial, which meant that their immune systems would have been greatly weakened. Second, several patients in this trial had soft-tissue sarcomas, which are generally poorly responsive towards immunomodulating therapies. Despite these factors, SQ3370 appears to have elicited an immunomodulatory response in these tumours.
What I like about this preprint
Click chemistry has gained traction in the field of chemical biology for several decades now—my last preLight was about a preprint by Nian and colleagues which uses a DNA-based ligand to improve copper-catalysed azide-alkyne cycloaddition (a type of click reaction). Now, this preprint by Srinivasan and co-workers demonstrates one of the earliest examples of the click reaction being used in medicine.
As Srinivasan and co-workers point out, the main advantage of the CAPAC platform lies in the use of chemical—as opposed to biological—methods to activate the protodrug. The reliance on biological factors to selectively release the active drug, such as conditions in the tumour microenvironment or the presence of certain enzymes, is susceptible to resistance arising from biological changes or amino acid mutations as the cancer progresses. Using a chemical method gives patients and their healthcare team alternative options to manage or even treat these intractable diseases.
Future directions
In their preprint, the authors discuss the use of the CAPAC platform in cancer treatment. Looking forward, I propose that a more ambitious goal should be considered by the biological community in applying this concept to the treatment of other challenging diseases.
One endeavour immediately springs to mind: antimicrobial resistance, which has been a growing problem for the past few decades. Often, many of the considerations in anticancer treatment—such as efficacy, selectivity, tolerability, and resistance associated with prolonged use—also apply to antimicrobials. If the authors’ CAPAC platform can reduce the toxicity in cancer treatment, then the same principle may apply in antimicrobial treatments.
What if we could selectivity target the sites of infection in patients, rather than treating patients systemically? This would reduce side effects associated with antimicrobial use, which can range from rashes to kidney injury. The selectivity would also decrease the risk of antimicrobial resistance. It may even be able to target the harmful microbes while sparing the commensal ones that are part of the body’s microbiome (like those found in the skin or gut), which may also help decrease the chance of opportunistic infections caused by more pathogenic bacteria.
Another technology that would enhance this platform is the development of click reactions. The chemistry underlying the work described in this preprint utilises the click reaction between tetrazine and trans-cyclooctene, an exceptionally fast reaction that does not require the use of metals. These properties make this click reaction particularly suitable for in vivo use. Discovering exceptionally fast chemical reactions that can take place in water—and tolerate a biological environment—will be essential for the development of faster bioorthogonal chemical methods.
Acknowledgements
Images created using Microsoft Powerpoint and BioRender.
doi: https://doi.org/10.1242/prelights.34587
Read preprintHave your say
Sign up to customise the site to your preferences and to receive alerts
Register hereAlso in the pharmacology and toxicology category:
Snake venom metalloproteinases are predominantly responsible for the cytotoxic effects of certain African viper venoms
Daniel Osorno Valencia
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
In vitro pharmacokinetics and pharmacodynamics of the diarylquinoline TBAJ-587 and its metabolites against Mycobacterium tuberculosis
Zhang-He Goh
preLists in the pharmacology and toxicology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
COVID-19 / SARS-CoV-2 preprints
List of important preprints dealing with the ongoing coronavirus outbreak. See http://covidpreprints.com for additional resources and timeline, and https://connect.biorxiv.org/relate/content/181 for full list of bioRxiv and medRxiv preprints on this topic
| List by | Dey Lab, Zhang-He Goh |
1
Drug use in special populations
Any drugs that are being used in special populations: Patients with liver and kidney failure, in paediatrics, in geriatrics, and in pregnant or lactating patients. Includes the discovery of factors that could potentially affect drug use in these special populations.
| List by | Zhang-He Goh |
Toxicology of toxicants, existing therapeutics, and investigational drugs
Preprints that describe the toxicology of environmental pollutants and existing and upcoming drugs. Includes both toxicokinetics and toxicodynamics, as well as technological improvements that will help in the characterisation of this field.
| List by | Zhang-He Goh |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |
Anticancer agents: Discovery and clinical use
Preprints that describe the discovery of anticancer agents and their clinical use. Includes both small molecules and macromolecules like biologics.
| List by | Zhang-He Goh |
Advances in Drug Delivery
Advances in formulation technology or targeted delivery methods that describe or develop the distribution of small molecules or large macromolecules to specific parts of the body.
| List by | Zhang-He Goh |











 (No Ratings Yet)
(No Ratings Yet)
2 years
Stig
Figure 1 is wrong?
SQL70 is modified with a tetrazine, not SQP33.
SQP33 is modifyet with a TCO, not a tetrazine.
But the end result is correct, that the TCO and SQL70 biopolymer is bound together TCO and tetrazine.