Subthreshold Voltage Analysis Demonstrates Neuronal Cell-Surface Sialic Acids Modulate Excitability and Network Integration
Posted on: 4 May 2020
Preprint posted on 8 April 2020
Neurons have a sweet tooth: How sugar molecules modify neuronal activity and network integration.
Selected by Berrak UgurCategories: neuroscience, physiology
Background
Neuronal membranes are coated with various sugar molecules called glycans (Figure 1) (Kleene and Schachner, 2004). These glycans are important for neuronal function and mutations that disrupt glycans cause a wide range of neurological disorders (Freeze et al., 2015). Among the vast variety of glycans, gangliosides that are sialylated glycosphingolipids have been associated with neuron excitability, axon stability and regeneration (Schnaar et al., 2014). However, how glycans, especially gangliosides, globally act to regulate neuronal electrophysiology is not well studied. To investigate how glycans contribute to neuronal network activity and integration, Kulkarni et al. performed voltage imaging in neurons treated with different sugar removing enzymes.
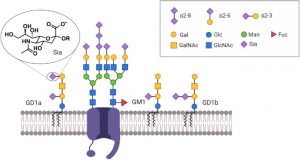 Figure 1: Scheme of neuronal cell membrane showing different types of glycans. Sialic acid (Sia), magnified, is negatively charged and is important for neuronal membrane charge. https://doi.org/10.1101/2020.04.07.030866
Figure 1: Scheme of neuronal cell membrane showing different types of glycans. Sialic acid (Sia), magnified, is negatively charged and is important for neuronal membrane charge. https://doi.org/10.1101/2020.04.07.030866
Key Findings:
The authors hypothesized that unique sialic acid modifications on neuronal membranes may selectively modify neuronal activity. To address this hypothesis, they used different sialidase enzymes that can either selectively cleave a specific sialic acid modification (2,3-linked sialic acid) or cleave a number of different sialic acid modifications. To record neuronal activity in bulk, the authors used a fluorescent voltage indicator called BeRST1 (Huang et al., 2015) that reports neuronal membrane potential with fast kinetics and relatively low noise. As previously suggested (Isaev et al., 2007), globally removing sialic acid in mouse hippocampal neurons resulted in a decrease in firing rate and population of live cells that are firing. Interestingly, selectively removing 2,3-linked sialic acid resulted in an increase in firing rate and populations of live cells that fire. This observation, the authors claim, may indicate a change in neuronal excitability as sialic acid may modify neuronal charge.
A method to assess if neuronal excitability is altered is to record subthreshold activity that may not be strong enough to trigger neuronal firing. The authors used the aforementioned voltage imaging technique coupled with a custom software library to delineate how sialidase treatment affects subthreshold activity. By performing various control experiments, the authors confirmed that (1) input from neighboring neurons generate a variance in subthreshold traces and (2) increased synapse formation leads to an increase in firing rate in mature neurons. Next, they examined selective removal of 2,3-linked sialic acid and concluded that it led to a decrease in action potential threshold but didn’t affect overall neuronal connectivity. However, promiscuously removing sialic acid resulted in a decrease in synchronized activity. The authors reasoned that asynchronous activity may be due to disrupted network integrity that leaves smaller connected neural networks (“islands”). To seek out if this is the case, the authors analyzed covariance between measured neurons based on the assumption that neurons receiving similar inputs will reflect similar activities (see Figure 2 for the model for “connectedness”). Consistent with a previous observation, selective removal of 2,3-linked sialic acid did not alter shared variance (covariance) between neurons indicating that network integration is not affected. In contrast, removing multiple sialic acid modifications led to reduction in shared variance between neurons indicating that global removal of sialic acid alters network connectivity. Overall, the authors show that different sialic acid modifications affect different neuronal properties.
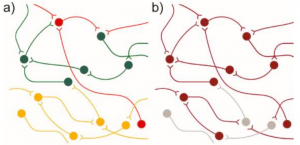 Figure 2: Model of network connectivity. Connected neurons behave similarly. Reduced connectivity may result in (a) islands of connected neurons, or b) some neurons losing their connection to the greater network. https://doi.org/10.1101/2020.04.07.030866
Figure 2: Model of network connectivity. Connected neurons behave similarly. Reduced connectivity may result in (a) islands of connected neurons, or b) some neurons losing their connection to the greater network. https://doi.org/10.1101/2020.04.07.030866
Take home messages:
- Removing neuronal cell surface sialic acid modification reduced firing of cultured hippocampal neurons.
- Selective removal of 2,3-linked sialosides led to increased action potential firing rate but did not affect network integration.
- Removing multiple sialic acid modifications led to a decrease in network connectivity
- It is likely that neurons utilize different sialylation subpopulations to fine tune neuronal activity.
What I liked about this story: First of all, I should say that I do not know much about glycobiology or the computational analyses performed in this preprint. However, I thought that a global measurement approach to understand how sialic acid modification alters neuronal function is a neat way to understand the effects of these sugar molecules. Considering how glycans are associated with various neurological disorders, this approach may also shed light on how sugars modify neural network connectivity in different disease models.
Remaining Questions
1) Have you tried measurements in neurons older than 12 DIV? I understand that they get unhealthy if you record from the start, is it possible to intervene and record at a later time point?
2) Is it possible to perform a similar analysis in hippocampal neurons obtained from a mouse model of Congenital disorders of glycosylation?
References:
Freeze, H.H., Eklund, E.A., Ng, B.G., Patterson, M.C., 2015. Neurological Aspects of Human Glycosylation Disorders. Annu. Rev. Neurosci. 38, 105–125. https://doi.org/10.1146/annurev-neuro-071714-034019
Huang, Y.-L., Walker, A.S., Miller, E.W., 2015. A Photostable Silicon Rhodamine Platform for Optical Voltage Sensing. J. Am. Chem. Soc. 137, 10767–10776. https://doi.org/10.1021/jacs.5b06644
Isaev, D., Isaeva, E., Shatskih, T., Zhao, Q., Smits, N.C., Shworak, N.W., Khazipov, R., Holmes, G.L., 2007. Role of extracellular sialic acid in regulation of neuronal and network excitability in the rat hippocampus. J. Neurosci. Off. J. Soc. Neurosci. 27, 11587–11594. https://doi.org/10.1523/JNEUROSCI.2033-07.2007
Kleene, R., Schachner, M., 2004. Glycans and neural cell interactions. Nat. Rev. Neurosci. 5, 195–208. https://doi.org/10.1038/nrn1349
Schnaar, R.L., Gerardy-Schahn, R., Hildebrandt, H., 2014. Sialic Acids in the Brain: Gangliosides and Polysialic Acid in Nervous System Development, Stability, Disease, and Regeneration. Physiol. Rev. 94, 461–518. https://doi.org/10.1152/physrev.00033.2013
doi: https://doi.org/10.1242/prelights.19980
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the neuroscience category:
PPARδ activation in microglia drives a transcriptional response that primes phagocytic function while countering inflammatory activation
Isabel Paine
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Self-renewal of neuronal mitochondria through asymmetric division
Lorena Olifiers
Also in the physiology category:
Resilience to cardiac aging in Greenland shark Somniosus microcephalus
Theodora Stougiannou
Trade-offs between surviving and thriving: A careful balance of physiological limitations and reproductive effort under thermal stress
Tshepiso Majelantle
Imaging cellular activity simultaneously across all organs of a vertebrate reveals body-wide circuits
Muhammed Sinan Malik
preLists in the neuroscience category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |
Also in the physiology category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |











 (No Ratings Yet)
(No Ratings Yet)