A therapeutic combination of two small molecule toxin inhibitors provides pancontinental preclinical efficacy against viper snakebite
Posted on: 29 May 2020
Preprint posted on 15 May 2020
Article now published in Nature Communications at http://dx.doi.org/10.1038/s41467-020-19981-6
Battling the serpent under the flower: potentially dispatching snakebite with a dual-antivenom combination
Selected by Zhang-He GohCategories: pharmacology and toxicology
Background of preprint
Snakebite, a neglected tropical disease (NTD) with high rates of morbidity and mortality globally, is currently considered a priority by the World Health Organisation (WHO). Unfortunately, while the WHO hopes to mitigate complications and death arising from snakebite by 2030, antivenoms to date suffer from a few key limitations that preclude their utility.
First, antivenoms currently consist of polyclonal immunoglobulins purified from the plasma or serum of large animals hyperimmunised with snake venoms. As such, antivenoms are generally not highly specific: many antibodies in the polyclonal mix do not bind to venom toxins, thus compromising antivenoms’ efficacies against envenoming by different snake species. The large doses of foreign immunoglobulins often needed for the antivenoms to be effective in turn contribute to a high incidence of adverse reactions. Furthermore, the accessibility of these antivenoms often present difficulties due to the need for a clinical setting for intravenous delivery and for cold chain transport and storage.
To circumvent these limitations, Albulescu et al. aimed to develop a therapeutic small molecule antivenom mixture effective against venoms from vipers found in different geographical regions. In this preprint, the authors explore three drugs for their efficacy against snake venom—the snake venom metalloproteinase (SVMP)-inhibitor marimastat, the phospholipase A2 (PLA2)-inhibitor varespladib, and the snake venom serine protease (SVSP) inhibitor (nafamostat)—and demonstrate the effectiveness of a combination of marimastat and varespladib against envenoming using a mouse model (Fig. 1).
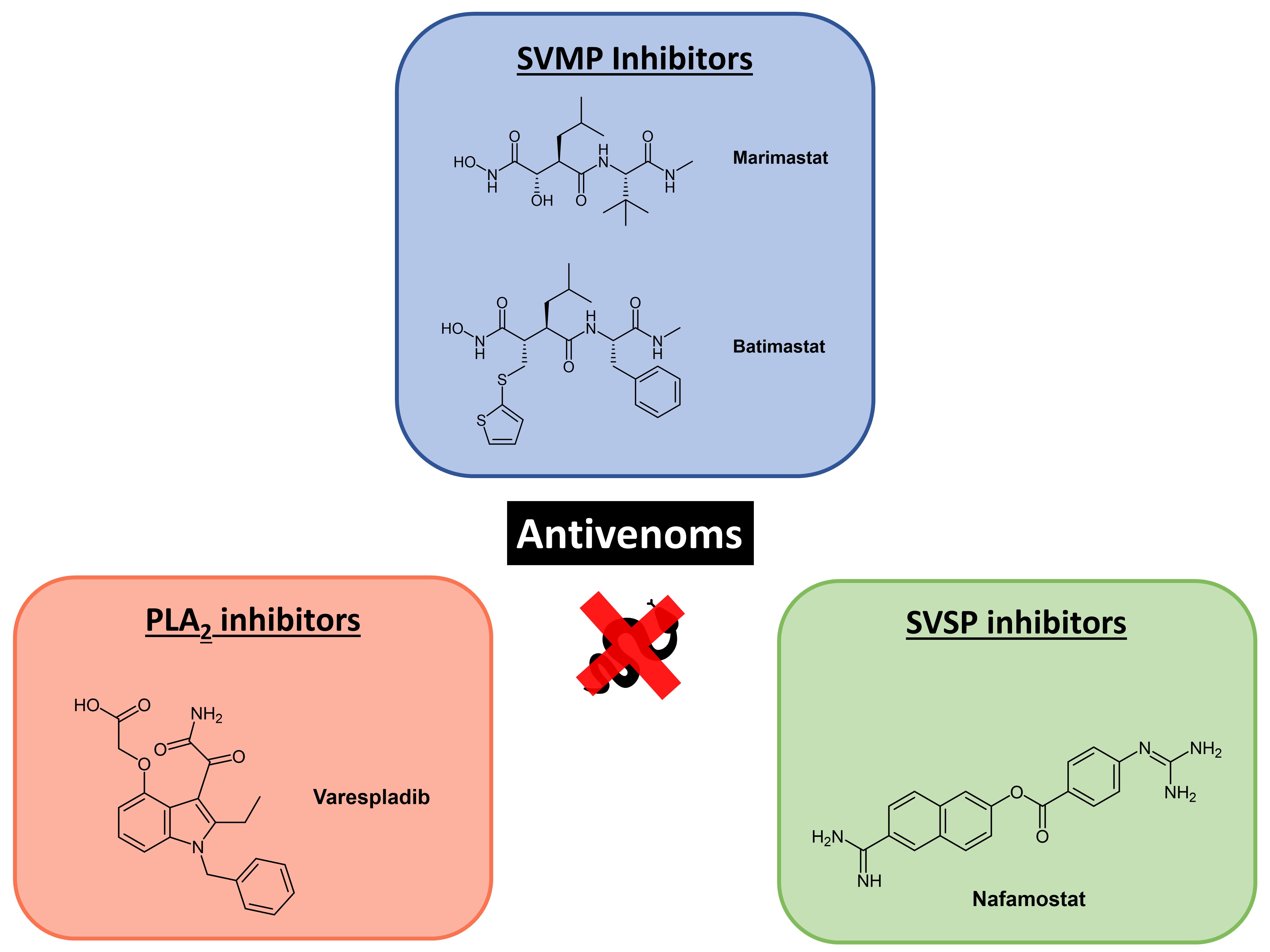
Figure 1. Potential antivenoms explored in this preprint.
Key findings of preprint
Albulescu et al. first tested two SVMP inhibitors, marimastat and batimastat, against venoms of snakes from geographically diverse regions (preprint Fig. 1) using an in vitro kinetic fluorogenic assay (preprint Fig. 2B-C) and a coagulation assay (preprint Fig. 3). The authors found that both antivenoms performed efficaciously, and ultimately selected marimastat over barimastat for further investigations due to marimastat’s superior pharmacokinetics [1] and toxicity profile.
The authors also identified a secondary uninhibited anticoagulant activity in Daboia russelii venom arising from PLA2 toxins, which make up 35% of toxins in D. russelii venom. This led them to investigate the use of PLA2-inhibitor varespladib against D. russelii venom fractions. Indeed, Albulescu et al. showed that a combination of varespladib and marimastat restored clotting profiles to that observed in the control (preprint Fig. S2B).
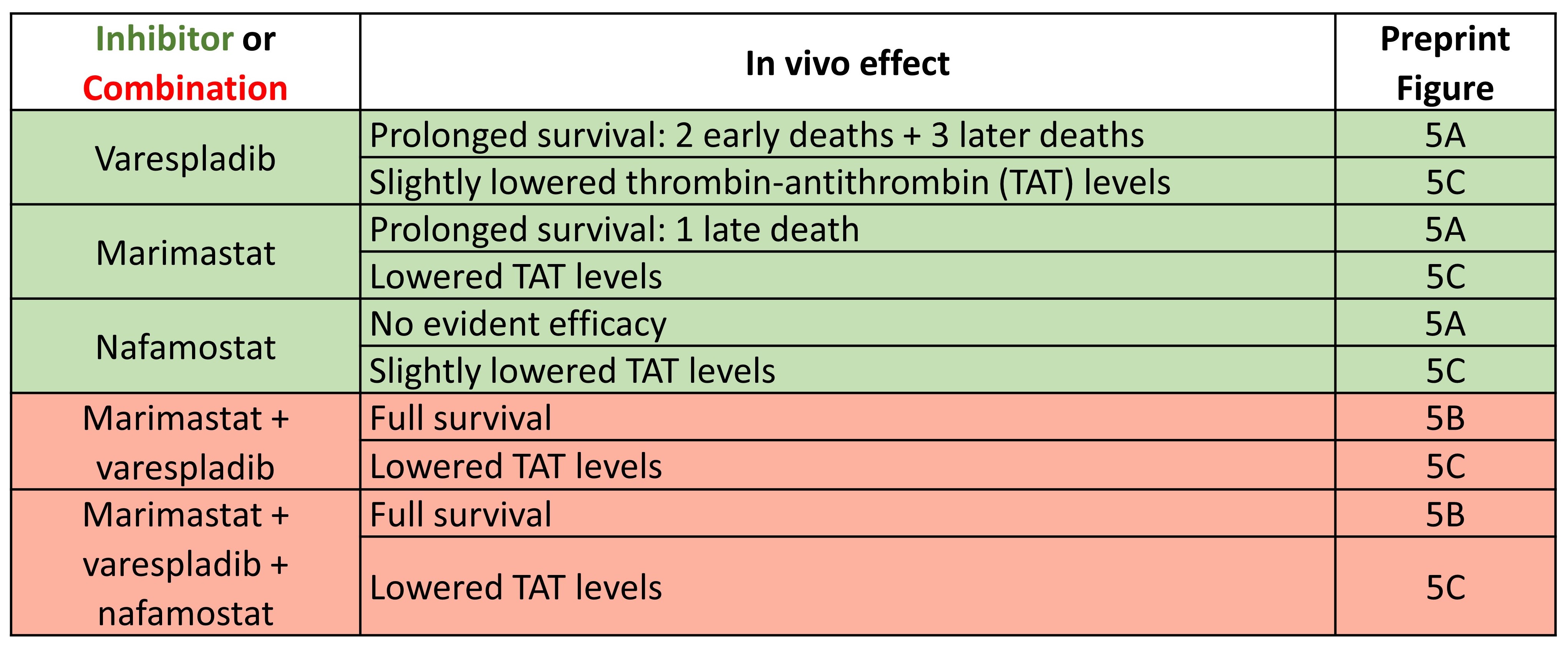
Having established the rationale for using PLA2 inhibitors (varespladib) and SVMP inhibitors (marimastat), Albulescu et al. further demonstrate the potential utility of SVSP inhibitors (nafamostat) as antivenoms using an in vitro assay. In subsequent tests of the efficacies of varespladib, marimastat, and nafamostat using an in vivo model of envenoming by Echis ocellatus (Table 1), the authors concluded that nafamostat does not contribute significantly to venom neutralisation.
Since nafamostat’s apparent lack of benefit would not balance out the risk of using another drug in combination, Albulescu et al. focussed on the combination of marimastat and varespladib for further development in a preclinical challenge-then-treat model of envenoming that better mimics real life scenarios. After demonstrating that the inhibitor combinations had no apparent adverse effects, the preprint authors showed that the marimastat-varespladib combination significantly protected against the venoms used in the in vivo challenge, as measured by overall survival and quantified TAT levels (preprint Fig. 6A-F).
Table 1. Efficacies of varespladib, marimastat, nafamostat individually or in combination in an in vivo model of envenoming.
What I like about this preprint: the future in an instant
I selected this preprint for two reasons. First, this preprint offers an alternative application of drug repurposing—using drugs that were originally developed for another disease in the treatment of another indication. Previously, I wrote about the utility of drug repurposing in antimicrobial discovery; this preprint shows how drug repurposing can also be applied to antivenom development. Many of the drugs explored in this study, such as varespladib, marimastat, and batimastat, had been advanced to Phase 2 or 3 in clinical trials, so their safety and pharmacokinetic profiles are already somewhat established.
Moreover, this preprint complements the authors’ recently-published work on using metal chelators as potential antivenoms [2]. Drug discovery is unpredictable and difficult: candidates tend to have a high failure rate as they move into clinical trials. Developing a large pool of candidates with different mechanisms of action will therefore help to boost the chance that an effective antivenom is ultimately realised—in all our nights and days to come.
References
[1] Millar AW, Brown PD, Moore J, Galloway WA, Cornish AG, Lenehan TJ, Lynch KP, Results of single and repeat dose studies of the oral matrix metalloproteinase inhibitor marimastat in healthy male volunteers, Br J Clin Pharmacol 45(1) (1998) 21-26.
[2] Albulescu L-O, Hale MS, Ainsworth S, Alsolaiss J, Crittenden E, Calvete JJ, Evans C, Wilkinson MC, Harrison RA, Kool J, Casewell NR, Preclinical validation of a repurposed metal chelator as an early-intervention therapeutic for hemotoxic snakebite, Science Translational Medicine 12(542) (2020) eaay8314.
doi: https://doi.org/10.1242/prelights.21419
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the pharmacology and toxicology category:
Snake venom metalloproteinases are predominantly responsible for the cytotoxic effects of certain African viper venoms
Daniel Osorno Valencia
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
In vitro pharmacokinetics and pharmacodynamics of the diarylquinoline TBAJ-587 and its metabolites against Mycobacterium tuberculosis
Zhang-He Goh
preLists in the pharmacology and toxicology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
COVID-19 / SARS-CoV-2 preprints
List of important preprints dealing with the ongoing coronavirus outbreak. See http://covidpreprints.com for additional resources and timeline, and https://connect.biorxiv.org/relate/content/181 for full list of bioRxiv and medRxiv preprints on this topic
| List by | Dey Lab, Zhang-He Goh |
1
Drug use in special populations
Any drugs that are being used in special populations: Patients with liver and kidney failure, in paediatrics, in geriatrics, and in pregnant or lactating patients. Includes the discovery of factors that could potentially affect drug use in these special populations.
| List by | Zhang-He Goh |
Toxicology of toxicants, existing therapeutics, and investigational drugs
Preprints that describe the toxicology of environmental pollutants and existing and upcoming drugs. Includes both toxicokinetics and toxicodynamics, as well as technological improvements that will help in the characterisation of this field.
| List by | Zhang-He Goh |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |
Anticancer agents: Discovery and clinical use
Preprints that describe the discovery of anticancer agents and their clinical use. Includes both small molecules and macromolecules like biologics.
| List by | Zhang-He Goh |
Advances in Drug Delivery
Advances in formulation technology or targeted delivery methods that describe or develop the distribution of small molecules or large macromolecules to specific parts of the body.
| List by | Zhang-He Goh |











 (No Ratings Yet)
(No Ratings Yet)