Design of smart antibody mimetics with photosensitive switches
Posted on: 30 December 2020
Preprint posted on 4 December 2020
Article now published in Advanced Biology at http://dx.doi.org/10.1002/adbi.202000541
Shining light on immunology’s dark corners: researchers design antibody mimetics with photosensitive switches
Selected by Zhang-He GohCategories: bioengineering, synthetic biology
Background of preprint: The way things were
Antibodies—special proteins of the immune system that recognise and bind to foreign species in the body—are incredibly useful tools in biology and medicine. Some of these antibodies found within the cell, known as intrabodies, have been engineered to respond to chemical and light changes.1-4 However, these intrabodies are currently limited in their slow response to these stimuli. To improve the intrabodies’ response to light, He et al. invented a pair of nanobodies that respond to light in seconds—light switches on the sunbody and switches off the moonbody (Fig. 1).
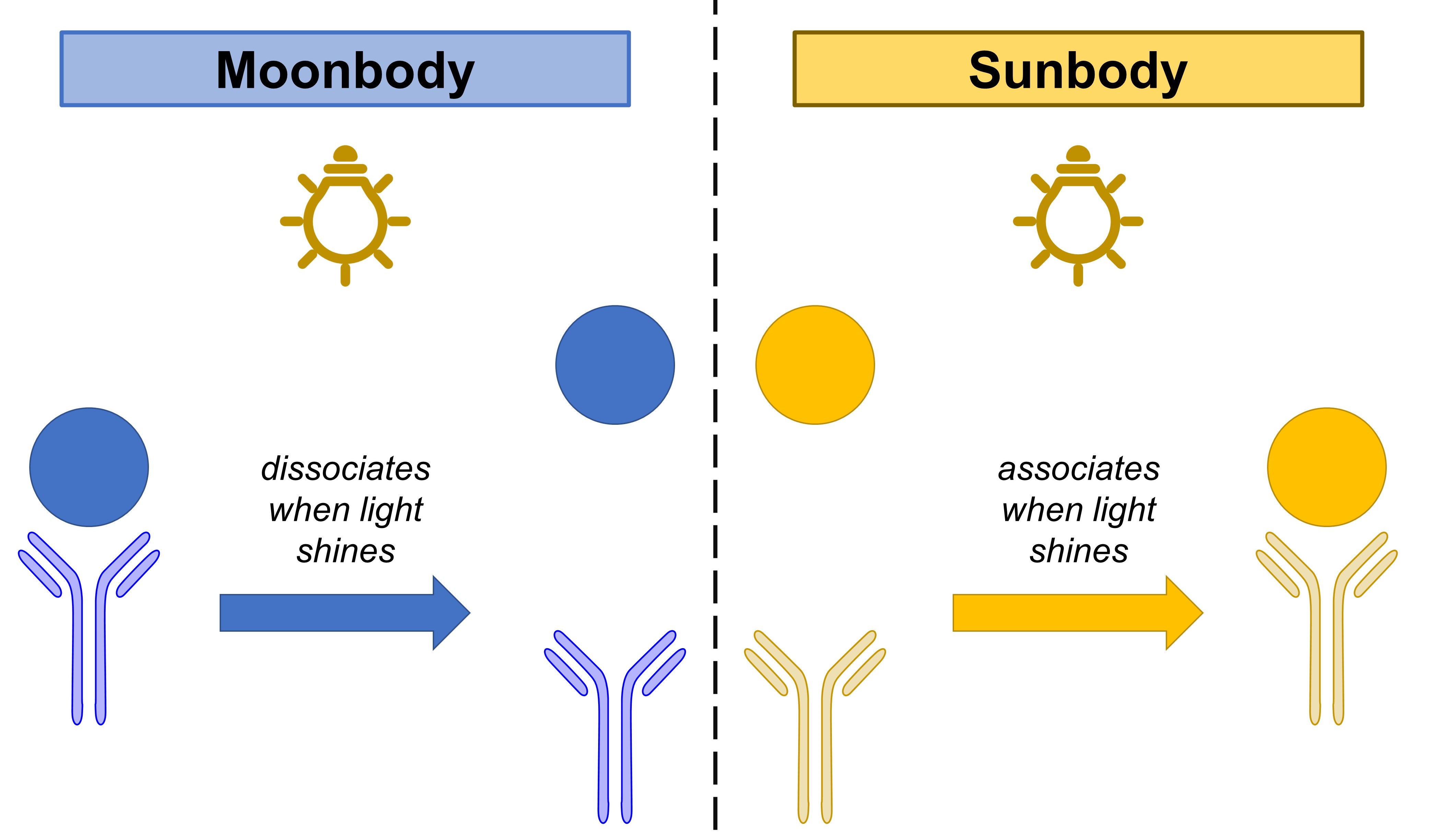
Figure 1. Moonbodies’ and sunbodies’ responses to light.
Key findings of preprint: shining in the laser light
He et al. first designed a moonbody by engineering a photoswitch called LOV2 into live cells. This was based on the hypothesis that light would induce changes in LOV2’s shape, allowing the authors to visualise the effect of light on the interaction between these intrabodies and its intended target—a nuclear envelope—in live cells. By varying the position of the LOV2 photoswitch, He et al. eventually made an intrabody construct that dissociated well from the nuclear envelope in the presence of light, which they named the “moonbody”. Using this approach, the authors also made two further moonbodies that targeted other parts of the cell.
Since the dissociation of these moonbodies could be induced simply by shining light on them, the authors sought to answer two questions: first, could the location of their activation be made specific by shining the light on one region while keeping the rest in the dark, and second, could activation be controlled by turning out the lights? Indeed, the authors even demonstrated spatial and temporal selectivity: by shining light on specific regions, they could target one field over another; and, by turning the light on and off, they could modulate the moonbodies’ conformations on a timescale of seconds.
Having constructed these moonbodies, He et al. used a similar approach to design “sunbodies”, nanobodies that could be activated by light. By combining the sunbody with the Fast Light- and Activity-Regulated Expression (FLARE) transcription factor system,5 the authors created the SolarFLARE platform for light-inducible transcriptional activation. He et al. used SolarFLARE to drive gene expression, stimulate downstream cellular effects like necroptosis, and even induce base editing using a photoactivable cytosine base editor.
What I like about this preprint: All at once, everything is different
I selected this preprint for its complementary and orthogonal nature with other recent advances1-2, 4 in modulating the conformations of biological constructs. By incorporating light-mediated ON- and OFF-switches into intrabodies, He et al. could regulate the interactions between these intrabodies and their targets. This is especially promising given that the shining of light is among the least invasive methods of regulating these changes; this strategy could potentially be used in conjunction with existing approaches—like small molecules—to effect more specific intracellular changes.
He et al. wrote that they hope that these photosensitive switches could be used in the “remote control of protein localisation, cell death, transcriptional programming, and precise base editing”. There are indeed potentially vast applications for these switches in the field of biology, ranging from boosting studies into cellular pathways to enabling new technologies in synthetic biology.
In addition to these upstream studies into cellular biology, it may be possible for these switches to be developed into therapeutic strategies. What particularly interests me is the possibility that these photosensitive switches could be used in therapeutic applications, ranging from drug delivery to being an actual part of the drug’s mechanism of action.
There is another reason behind highlighting this preprint to wrap up 2020. It has been an especially difficult year for everyone; the scientific community, too, has been greatly affected. Yet, it has also been encouraging to see how researchers have made significant scientific progress—especially in healthcare-related areas like biology, chemistry, and engineering. In Disney’s Tangled (2011), Rapunzel sings that the world has shifted, and everything is different. Just like in 2020. How apt, then, to celebrate our achievements in overcoming these challenges we have faced in the past year by highlighting a preprint about light.
Open questions
- What are some immediate implications for biologists given this work? Is there an area you are especially keen to see being studied using your strategy?
- You wrote about personalised medicine in your conclusion. Could you tell us more about these beverage-switchable antibodies and how they work?
- Do the sunbodies and moonbodies operate at specific wavelengths of light? What is the range of wavelengths that they can tolerate?
- Did the intensity of the light affect how quickly the sunbodies and moonbodies react to the stimuli?
References
- Yu, D.; Lee, H.; Hong, J.; Jung, H.; Jo, Y.; Oh, B.-H.; Park, B. O.; Heo, W. D., Optogenetic activation of intracellular antibodies for direct modulation of endogenous proteins. Nature Methods 2019, 16 (11), 1095-1100.
- Gil, A. A.; Carrasco-López, C.; Zhu, L.; Zhao, E. M.; Ravindran, P. T.; Wilson, M. Z.; Goglia, A. G.; Avalos, J. L.; Toettcher, J. E., Optogenetic control of protein binding using light-switchable nanobodies. Nature Communications 2020, 11 (1), 4044.
- Carrasco-López, C.; Zhao, E. M.; Gil, A. A.; Alam, N.; Toettcher, J. E.; Avalos, J. L., Development of light-responsive protein binding in the monobody non-immunoglobulin scaffold. Nature Communications 2020, 11 (1), 4045.
- Farrants, H.; Tarnawski, M.; Müller, T. G.; Otsuka, S.; Hiblot, J.; Koch, B.; Kueblbeck, M.; Kräusslich, H.-G.; Ellenberg, J.; Johnsson, K., Chemogenetic Control of Nanobodies. Nature Methods 2020, 17 (3), 279-282.
- Wang, W.; Wildes, C. P.; Pattarabanjird, T.; Sanchez, M. I.; Glober, G. F.; Matthews, G. A.; Tye, K. M.; Ting, A. Y., A light- and calcium-gated transcription factor for imaging and manipulating activated neurons. Nature Biotechnology 2017, 35 (9), 864-871.
doi: https://doi.org/10.1242/prelights.26691
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the bioengineering category:
A Novel Chimeric Antigen Receptor (CAR) - Strategy to Target EGFRVIII-Mutated Glioblastoma Cells via Macrophages
Dina Kabbara
Human pluripotent stem cell-derived macrophages modify development of human kidney organoids
Theodora Stougiannou
Matrix viscoelasticity regulates dendritic cell migration and immune priming
Roberto Amadio
Also in the synthetic biology category:
Enzymatic bromination of native peptides for late-stage structural diversification via Suzuki-Miyaura coupling
Zhang-He Goh
Enhancer cooperativity can compensate for loss of activity over large genomic distances
Milan Antonovic
Discovery and Validation of Context-Dependent Synthetic Mammalian Promoters
Jessica L. Teo
preLists in the bioengineering category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Advances in microscopy
This preList highlights exciting unpublished preprint articles describing advances in microscopy with a focus on light-sheet microscopy.
| List by | Stephan Daetwyler |
Also in the synthetic biology category:
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |
Advances in Drug Delivery
Advances in formulation technology or targeted delivery methods that describe or develop the distribution of small molecules or large macromolecules to specific parts of the body.
| List by | Zhang-He Goh |











 (No Ratings Yet)
(No Ratings Yet)