Drug prescription pattern in European neonatal units
Posted on: 17 November 2018 , updated on: 29 September 2019
Preprint posted on 5 November 2018
Baby’s first steps: The association of drug use in European neonatal units with geographic region and gestational age
Selected by Zhang-He GohCategories: pharmacology and toxicology
Background of preprint
Drugs are extensively used in neonates to reduce morbidity and mortality, as well as improve their health. However, despite the vulnerable profile of these patients, their drug use not been well studied. The primary difficulty in conducting this research lies in methodological differences across studies done between countries, as the resultant inter-country variation makes the identification of any large-scale trends particularly challenging.
To bridge this research gap, Mesek et al. conducted a sub-study under the auspices of the European Study of Neonatal Exposure to Excipients (ESNEE), a pan-European project. The authors formulated two hypotheses: first, that gestational age (GA) would influence drug use regardless of region, because underlying conditions depend on GA; and second, that drug use should not depend on geographic region, for the same reason.
Therefore, the authors conducted this study, which, like most clinical studies, revolved around a framework containing a trio of core analyses following data collection. First, the baseline characteristics of the sample gathered from the study population were studied to ensure that the analysis would be appropriate, and the descriptive statistics were presented in Table 1 of the study. Then, the data were analysed to test for differences that answered the research question. Finally, interesting or unexpected trends were picked out and the causes underlying these observations were attributed to various factors.
Key findings of preprint
(A) Analysis of participants’ baseline characteristics
In their study, Mesek et al. first presented the baseline characteristics of their sample of the study population in Table 1 of their preprint, which described the distribution of study participants based on geographic region—referring to countries that were broadly classified into East, North, South, and West Europe. A similar analysis was conducted for the distribution of the study population according to the level of maturity as measured by GA—preterm neonates were classified into “extremely preterm”, “very preterm”, “late preterm”, and “term”. The results of this latter analysis were presented in Table 2 of the preprint. Interestingly, differences were observed in the baseline characteristics of the participants across regions and across GA groups in this study. This was itself a subject for further discussion. In a vast majority of clinical studies, we would not expect significant differences to be observed in participants’ baseline characteristics due to their carefully controlled nature.
(B) Comparison of drug use between different GA groups and geographic regions
Mesek et al. were interested in the relationships between three factors: drug use, GA group, and geographic region (Figure 1).
Figure 1. Relationships analysed by Mesek et al. in their preprint.
Comparison of drug use across different GA groups. The authors found a significant downward trend in drug use with increasing GA, with prescription rates among extremely preterms two times higher than term neonates. A few key trends were highlighted:
- Caffeine was very commonly used by extremely preterms and very preterms, though this rate decreased steeply for late preterms and term neonates.
- Multivitamins were more commonly used among preterms than term neonates.
- Vitamin D was used among preterms with similar frequency, but less so among term neonates.
- Anti-infectives were used most commonly among extremely preterms.
- Extremely preterms were more likely to receive nervous system and cardiovascular drugs than all other groups.
- Extremely preterms were more likely to receive drugs for blood and blood-forming organs, as well as for the alimentary system, compared to late preterm and term neonates.
- Extremely preterms were more likely to receive anti-infectives compared to late preterms.
- Extremely preterms were more likely to receive respiratory system drugs compared to term neonates.
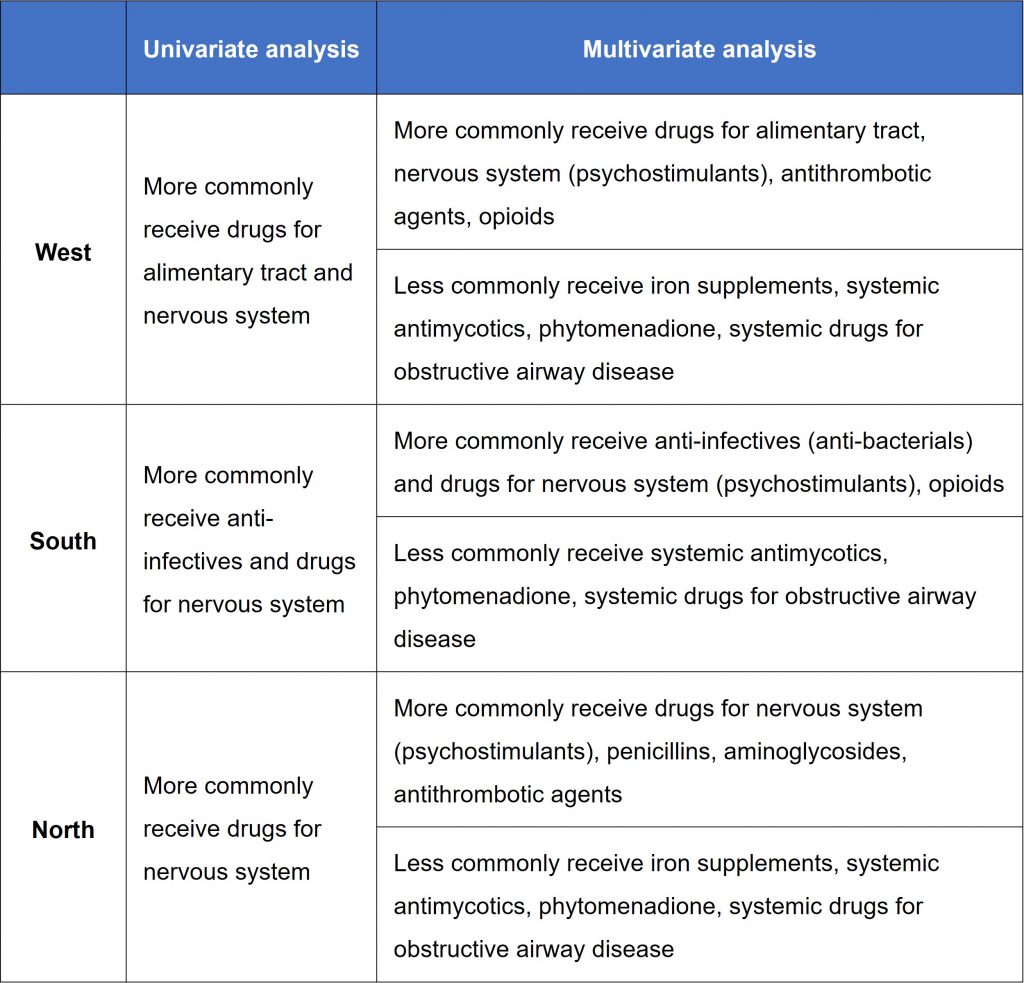
Comparison of drug use across different geographic regions. Mesek et al. highlighted West Europe, which had a significantly higher prescription rate than North, East, and South Europe. The authors then conducted both univariate and multivariate analyses, revealing significant geographic variations in all the most commonly used Anatomical Therapeutic Chemical (ATC) classes, except for cardiovascular drugs. Key trends are summarised in Table 1.
Table 1. Key trends observed in drug use across different geographic regions, compared to East Europe.
Comparison of drug use across different GA groups. Mesek et al. made two key observations. First, the use of anti-infectives was the highest in extremely preterm and term neonates. This was ascribed to separate factors. Extremely preterm neonates tend to have higher rates of infection; while in term neonates, infection is usually the most common cause of hospitalisation. Second, drug use generally declined in patients with older GA; this was attributed to recommendations in guidelines of clinical consensus established by learned societies.
(C) Attribution of interesting or unexpected trends to various causes
Comparison of drug use across different geographic regions. The authors’ findings in this analysis can also be subdivided into two groups: anti-infectives, and other drugs. The prescribing pattern of anti-infectives was attributed to both differences in microbial resistance rates and geographically-differing medical practices. In contrast, the causes of differences observed in the prescribing patterns of other drugs were not as clear. Mesek et al. propose that these were caused by conflicting evidence arising from the dearth of reliable studies and obsolete clinical practice that may not have incorporated the latest results derived from clinical research.
What I like about this preprint
I selected this preprint for two reasons. First, as Mesek et al. point out, it is “the largest multi-country survey of drug use covering neonatal units across Europe and neonates of all GA groups”. Indeed, multicentre collaborations are difficult to conduct, but are tremendously valuable in two ways: they have significant amounts of statistical power, and they also allow for rigorous comparisons between centres in different regions due to the very nature of the study design.
The second reason for choosing this preprint is its significance in the field of clinical research. Due to its power and its multicentre nature, this study provides a compelling basis on which future research can be prioritised. It refines existing research gaps in drug use in neonates into more focussed areas which can be more rigorously studied on a practical level. For example, Mesek et al. note that the use of caffeine in reducing bronchopulmonary dysplasia in preterms with apnea has been met with conflicting evidence. Having identified these smaller, more specific research gaps, future studies can focus on collecting more specific data that would be better able to answer these research questions.
Future directions
Future directions for this work will likely focus on mitigating the limitations highlighted by Mesek et al.:
- Voluntary participation may have led to data being collected that do not fully represent the countries that participated in this trial. Some countries were also excluded altogether.
- The definition of late preterms could have influenced results if this population behaved as “very preterms” or as late preterms.
- Postnatal age could have been a potential confounder.
- Rarely used drugs may not have been captured representatively due to the nature of the study design.
Therefore, further efforts will focus on changing the study design, collecting more data on variables, and recruiting a greater number of people. To achieve this, more inter-country coordination and collaboration, with major European countries participating in this multicentre effort. Moreover, the methodology used in data collection may also be further standardised to reduce variation introduced from the experimental design.
At the same time, other auxiliary work that would be immensely useful to this study may also be done. These include studies that focus on not just prescribing or drug use patterns, but also healthcare professionals’ opinions on neonatal care. For instance, the frequency with which physicians and pharmacists conform to guidelines published by learned societies could be assessed and reasons for deviations documented to better elucidate the roles of cross-regional cultural factors in influencing healthcare professionals’ clinical decisions. At the same time, in-house guidelines on neonatal care from various healthcare institutions across the four regions could also be juxtaposed to shed further insight on the trends that have been observed in this work. All in all, the baby steps formed by this study point toward further areas for improvement on clinical guidelines for neonates. Hopefully, this will lead healthcare professionals to achieve a standard of therapy that can be customised to each individual patient profile.
Questions for authors
- One reason for differences in regional use of anti-infectives is the microbial resistance rate. Is it also possible that the causative microorganisms themselves differ? For example, the antibiotic to be used will often differ depending on whether the causative bacteria are gram-positive or gram-negative. Hence, how much of this variation in the prescribing pattern is likely to be caused by differences in the causative microorganisms themselves, rather than the resistance rates?
- How were the geographical regions defined? Which factors decided how the countries were grouped into the respective geographical regions?
doi: https://doi.org/10.1242/prelights.5620
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the pharmacology and toxicology category:
Snake venom metalloproteinases are predominantly responsible for the cytotoxic effects of certain African viper venoms
Daniel Osorno Valencia
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
In vitro pharmacokinetics and pharmacodynamics of the diarylquinoline TBAJ-587 and its metabolites against Mycobacterium tuberculosis
Zhang-He Goh
preLists in the pharmacology and toxicology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
COVID-19 / SARS-CoV-2 preprints
List of important preprints dealing with the ongoing coronavirus outbreak. See http://covidpreprints.com for additional resources and timeline, and https://connect.biorxiv.org/relate/content/181 for full list of bioRxiv and medRxiv preprints on this topic
| List by | Dey Lab, Zhang-He Goh |
1
Drug use in special populations
Any drugs that are being used in special populations: Patients with liver and kidney failure, in paediatrics, in geriatrics, and in pregnant or lactating patients. Includes the discovery of factors that could potentially affect drug use in these special populations.
| List by | Zhang-He Goh |
Toxicology of toxicants, existing therapeutics, and investigational drugs
Preprints that describe the toxicology of environmental pollutants and existing and upcoming drugs. Includes both toxicokinetics and toxicodynamics, as well as technological improvements that will help in the characterisation of this field.
| List by | Zhang-He Goh |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |
Anticancer agents: Discovery and clinical use
Preprints that describe the discovery of anticancer agents and their clinical use. Includes both small molecules and macromolecules like biologics.
| List by | Zhang-He Goh |
Advances in Drug Delivery
Advances in formulation technology or targeted delivery methods that describe or develop the distribution of small molecules or large macromolecules to specific parts of the body.
| List by | Zhang-He Goh |











 (No Ratings Yet)
(No Ratings Yet)