Molecular dynamics simulations disclose early stages of the photo-activation of cryptochrome 4
Posted on: 4 June 2018 , updated on: 6 June 2018
Preprint posted on 17 May 2018
Article now published in New Journal of Physics at http://dx.doi.org/10.1088/1367-2630/aad70f
Birds use a magnetic compass for orientation during their amazing migratory journeys. This study highlights the involvement of candidate regions of the putative Cry4 receptor molecule in magnetoreception related signal transduction.
Selected by Miriam LiedvogelCategories: animal behavior and cognition, biophysics
Background
Birds are able to use the Earth’s magnetic field as reference cue for orientation during their amazing migratory journeys. From behavioural experiments we know what characteristics of the magnetic field the birds are using, but we still don’t understand how birds are sensing the magnetic field.
The magnetic compass in migratory birds has been shown to be light-dependent, and theoretical physicists have suggested the reaction could be based on a radical pair reaction, where the ratio of singlet and triplet transient states of a radical pair could be indicative for the orientation of that molecule within the magnetic field. Cryptochromes have been suggested as promising candidates as they are the only known photosensitve proteins in the vertebrate eye that have the potential to form radical pairs. In theory, cryptochromes (Cry) in the bird’s eye undergo a specific chemical reaction that is governed by the direction of the Earth’s magnetic field, and could provide a signal for orientation.
Cryptochromes come in different flavours and are members of a multigene family of blue light photoreceptors. In birds, four different cryptochromes have been identified (Cry1a and Cry1b that are different splicing variants, Cry2 and Cry4) – but which of them could potentially act as the magnetosensor? Cryptochrome 4 stands out as the most promising candidate and receives most attention in this skating exhibition, because it comes with specific features that distinguish it clearly from other family members.
Cry4 has been detected in the retina of several bird species and is particularly suited as (unlike the other mentioned family members) expression levels are constant throughout the day and independent of a circadian rhythm. Importantly, expression levels are higher during the migratory season compared to periods of the year when these birds do not engange in oriented long-distance flights, which clearly supports its role as putative magnetosensor. Cry4 shows high affinity to bind the photacitve flavin cofactor (FAD) and undergoes light-dependent structural changes in the C-terminal end, which are investigated focally here. The protein structure of this promising magnetoreceptor molecule remains to be solved, but a homology model for Cry4 from the European robin (ErCry4), an iconic and well described study species in the field of bird migration, has been established.
Key findings
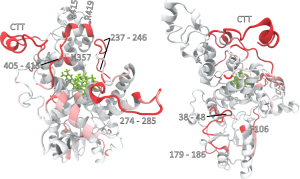
Here the authors use this homology model to simulate structural reorganisations that accompany the photoreduction of the flavin cofactor and are able to demonstrate that photo-activation of the hypothesized magnetoreceptor molecule induces large-scale conformational changes on very short timescales. Excitingly, these molecular dynamics simulations disclose early stages of the photo-activation of cryptochrome 4. Specifically, the photoreduction leads to the release of the C-terminal region, accompanied by structural rearrangements close to the binding site for the photacitve flavin cofactor binding site.
The authors highlight strengths and shortcomings of different modelling approaches and graphical representations that are used to identify common motifs of these mobile sites. Using a graph-based approach to describe the conformational dynamics of the potential magnetoreceptor protein upon photoactivation, the authors are able to show that these rearrangements appear to expose potential phosphorylation sites that have the potential to be functionally coupled to photoactivation. Although based on simulation data only so far, this provides the exciting possibility that these phosphorylation sites could have a function in modulating/deactivating the photo-transduction cascade.
The authors speculate this could be realised in a manner similar to the deactivation of rhodopsin (another retinal photopigment), where photoexcitation triggers conformational changes that lead to phosphorylation and subsequent regulation of downstream transduction steps in the visual process.
Summary and future prospects This paper reveals the identification of residual sites in the hypothesized magnetoreceptor protein Cry4 of migratory robin that undergo conformational changes triggered by photoactivation. Specifically, the authors identify the most promising sites to be located at the C-terminal tail of the protein or motifs closely coupled to that region. Importantly, these results identify candidate regions that are potentially involved in signal transduction related to magnetoreception and pave the avenue for experimentally testing these major regulators in subsequent experiments, possibly by targeted molecular manipulation of the focal sites. These exciting findings clearly call for support by experimental evidence from analogous studies on the crystal structures once available.
What I like about this work Understanding how birds perceive the Earth’s magnetic field and use this information to orient during their fascinating long migratory journeys is one of the key unresolved mysteries in sensory biology. This study capitalises on the recently published homology model of the candidate receptor molecule Cry 4 from European robins to identify key regulatory candidate regions and predict their involvement in magnetic compass orientation related signal transduction. Understanding the phenomenon of magnetoreception undoubtably needs a cross-disciplinary and highly integrative approach. This study provides a perfect example how theoretical prediction provide the necessary avenue for experimentalists and behavioural biologists to join in and design the diagnostic tests to challenge these findings in analogous studies and/or with complementary approaches.
And one question to the authors – and yes, I am asking for gut feeling here The transient radical pair is the one that is the one that is affected by and thus the one translating magnetic information into an oriented behavior. Here the the focus is on a radical pair state that is photoinduced from the dark state protein with the fully oxidised FAD cofactor. What is your feeling here – do you think this is “it”, i.e.the hero radical pair that runs for magnetoreceptor, or is it the fully reduced and then putatively reoxidise Cry4?
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the animal behavior and cognition category:
Morphological variations in external genitalia do not explain the interspecific reproductive isolation in Nasonia species complex (Hymenoptera: Pteromalidae)
Stefan Friedrich Wirth
Trade-offs between surviving and thriving: A careful balance of physiological limitations and reproductive effort under thermal stress
Tshepiso Majelantle
Responses to conflicting binocular stimuli in mouse primary visual cortex
Maitri Manjunath
Also in the biophysics category:
Active flows drive clustering and sorting of membrane components with differential affinity to dynamic actin cytoskeleton
Teodora Piskova
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
Mitochondrial Fatty Acid Oxidation is Stimulated by Red Light Irradiation
Rickson Ribeiro, Marcus Oliveira
preLists in the animal behavior and cognition category:
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Bats
A list of preprints dealing with the ecology, evolution and behavior of bats
| List by | Baheerathan Murugavel |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Also in the biophysics category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
66th Biophysical Society Annual Meeting, 2022
Preprints presented at the 66th BPS Annual Meeting, Feb 19 - 23, 2022 (The below list is not exhaustive and the preprints are listed in no particular order.)
| List by | Soni Mohapatra |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
Biophysical Society Meeting 2020
Some preprints presented at the Biophysical Society Meeting 2020 in San Diego, USA.
| List by | Tessa Sinnige |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Biomolecular NMR
Preprints related to the application and development of biomolecular NMR spectroscopy
| List by | Reid Alderson |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |











 (No Ratings Yet)
(No Ratings Yet)