Neuregulin-1 exerts molecular control over axolotl lung regeneration through ErbB family receptors
Posted on: 23 April 2018
Preprint posted on 1 February 2018
All for one and one for all: Axolotl lungs regenerate through an NRG1-ErbB dependent compensatory response that involves cells throughout the lung and few rounds of division, rather than a highly-proliferative localized blastema
Selected by Alberto Rosello-DiezCategories: molecular biology
Background
While some human organs can undergo compensatory growth after amputation or damage, the process is extremely slow, and learning the fundamental aspects of this process in order to speed it up would have a strong impact on regenerative medicine. A priori, there are at least two conceivable mechanisms by which organs can recover their lost mass: 1) epimorphic regeneration, whereby some cells near the wound dedifferentiate and form a highly-proliferative blastema (e.g. regenerating limbs in amphibians and fins zebrafish); 2) compensatory growth of the organ as a whole, such that the mass is recovered but the original shape is not (e.g. liver in humans). Jensen and colleagues use the axolotl salamander to study lung regeneration, a process relatively unexplored outside mammalian models. Their molecular analysis focuses on the ErbB family of receptors and its activation of YAP signalling, both of which are important for pulmonary tissue proliferation. In particular, the ligand NRG1, the receptors ErbB2/4 and the YAP target HoxA1 are the usual suspects involved in the proliferative response [1-3].
Key findings
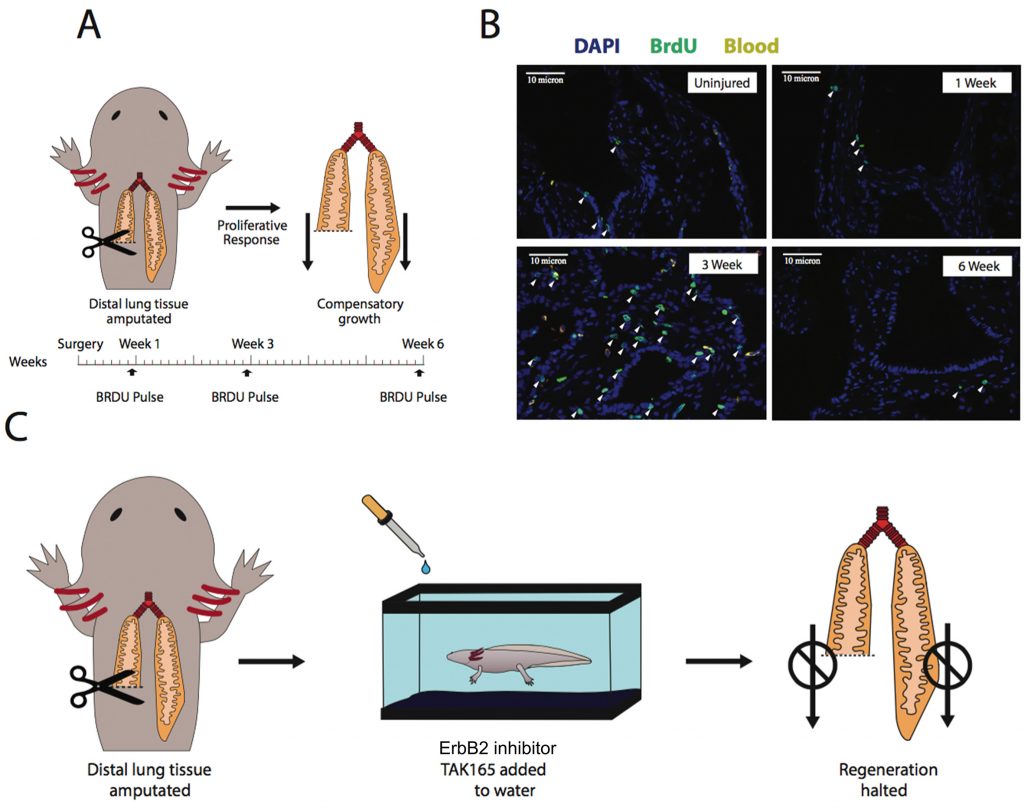
- Amputation of one third of one of the lungs leads to a rapid wound closure, with inflammation receding 7 days post amputation (dpa).
- There is a strong proliferative response as early as 7 dpa, but strikingly it is not restricted to the amputated region, but rather a whole-organ response, including the contralateral lung (Fig. 1A, B).
- While lung length is not overly recovered, lung volume is, and therefore the overall shape of the lung is altered. After 8 weeks, the amputated lung catches up to the control one in volume.
- Proliferative cells undergo only a few rounds of cell division, and tracking those cells suggests they give rise only to cells of their own cell type. If confirmed by marker expression studies, this would indicate that, as in rodent lungs [4], there is no a restricted population of multipotent stem cells that carries out the whole regenerative response. This is distinct from limb regeneration in the axolotl, which despite being quite lineage-restricted [5], works through the formation of a highly proliferative blastema where progenitor cells accumulate and proliferate actively.
- NRG1, ErbB4 and HoxA1 are upregulated in the injured lungs, suggesting they are responsible for the proliferative response. In fact, systemic inhibition of ErbB2 (which binds NRG1 and heterodimerizes with ErbB4) abolishes the proliferative response in injured lungs (Fig. 1C). Moreover, injection of NRG1 in uninjured lungs activates proliferation and a similar molecular response as seen upon amputation, and these responses depend on ErbB2 signalling.
What I like about this preprint
The study of Jensen and colleagues (understandably foundational as it is the senior thesis work of the first author) reveals that not all organs regenerate through the same mechanism in axolotl, and opens up a new set of questions to be explored in the future. It is surprising and refreshing that new basic discoveries are still possible in such an long-established model.
Pending questions
- Despite proliferation being activated in both the injured and contralateral lung, the injured one eventually catches up to the intact one in volume. Is this due to mechanical factors, such as there being more space available for the resected lung? And what happens to the extra cells in the uninjured lung?
- The authors suggest that inflammation plays a role to maintain the proliferative cells undifferentiated through repression of EGFR (ErbB1). Have the authors considered using antagonists of IL1B signalling after amputation, or agonists in combination with NRG1 treatment?
- The fact that NRG1 is able to induce proliferation of lung cells in the absence of amputation suggests that mass reduction is not required for the compensatory response to happen. If this is the case, I wonder how do lung cells ‘know’ when they have to stop proliferating –in other words, how do they know when the lung has been repaired?
Related research
- Liu et al. 2009. Exp. Lung Res.
- Haskins et al. 2014. Sci. Signal.
- Liu et al. 2015. Mol. Cell. Biol.
- Kotton et al. 2014. Nature Medicine.
- Kragl et al. 2009. Nature.
Sign up to customise the site to your preferences and to receive alerts
Register hereAlso in the molecular biology category:
A drought stress-induced MYB transcription factor regulates pavement cell shape in leaves of European aspen (Populus tremula)
Jeny Jose
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
preLists in the molecular biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |











 (No Ratings Yet)
(No Ratings Yet)