Oligodendrocyte precursor cells engulf synaptic inputs in an experience- and microglia-dependent manner
Posted on: 24 May 2022 , updated on: 9 June 2022
Preprint posted on 10 February 2022
Not just myelination: Oligodendrocyte progenitor cells (OPCs) also engulf synapses and participate in circuit remodeling using a mechanism that depends on microglia
Selected by Meghan WynneCategories: neuroscience
Background
The last couple of decades have seen an explosion in interest in the functional roles played by the brain’s glial cells (Allen and Lyons, 2018; Barres, 2008). For instance, it has been shown that microglia and astrocytes play key roles in the refinement of maturing brain circuits by phagocytosing excess synapses (Chung et al., 2013; Schafer et al., 2012; Vainchtein et al., 2018). Despite the growing interest in glial cell biology, there has been little research exploring functions served by oligodendrocyte progenitor cells (OPCs) beyond their known role in serving as precursor cells for oligodendrocytes, which myelinate axons. OPCs persist in the adult brain long after the production of new oligodendrocytes has waned and mature myelination patterns have been adopted(Clayton and Tesar, 2021), and also respond to cues from both neurons and glia in differentiation and myelination, and form synapses with neurons (Barres and Raff, 1993; Bergles et al., 2000; Boulanger and Messier, 2017; Gibson et al., 2014; Pease-Raissi and Chan, 2021). These properties suggest OPCs play important roles across the lifespan of the brain that have yet to be characterized.
Key findings
The authors focused on a visual circuit connecting the thalamus to the cortex. This particular thalamocortical circuit consists of inputs from the dorsal lateral geniculate nucleus (dLGN) of the thalamus synapsing onto neuronal targets in layer 4 of the primary visual cortex (V1) (Fig. 1 below). This circuit has been established as a model for the study of experience-dependent plasticity since it undergoes a period of heightened synaptic refinement dependent on visual experience during the third week of life in the mouse. This vision-dependent synapse elimination also continues into adulthood (Cheadle et al., 2020; Mataga et al., 2004).
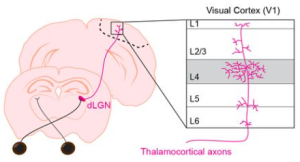
The authors used immunofluorescence and advanced microscopy techniques to study how OPCs and microglia interacted with synaptic inputs during synaptic refinement in the developing and adult brain, examining both ex vivo slices and unanesthetized mice in vivo. Intriguingly, they observed phagocytosis of thalamic inputs to V1 by OPCs, as the presynaptic marker vglut2 was localized inside many OPCs and directed to lysosomes, presumably for degradation. This synaptic engulfment by OPCs was increased when mice reared in the dark during a critical developmental period were re-exposed to light, showing OPC synaptic engulfment is responsive to experience-driven neural activity. Interestingly, the authors showed that the light deprivation paradigm altered the nature of physical contacts between OPCs and microglia, as sensory deprivation shifted the contact site to more distal ends of OPC arbors. Moreover, pharmacological ablation of microglia during the light deprivation paradigm abolished the increase in OPC synaptic engulfment that previously occurred with light re-exposure. The authors also showed that OPC synaptic engulfment occurred in the adult mouse brain at P90. Together, the work shows a novel function for OPCs in synapse elimination across the lifespan of the brain, including in response to sensory experience, that depends on a signaling mechanism from microglia.
Why I liked this preprint
Phagocytosis of synapses by astrocytes and microglia is a well-studied function of glia and its dysregulation is thought to influence neurodevelopmental, neuropsychiatric, and neurodegenerative disease(Bolton et al., 2022; Chung et al., 2016; Hong et al., 2016; Yilmaz et al., 2021). While OPCs are thought to contribute to brain diseases through roles in myelination (Clayton and Tesar, 2021) this work opens up the possibility that OPCs may also affect pathogenesis of brain diseases through regulation of synapse elimination. In showing that OPCs contribute to circuit refinement beyond regulation of myelination, this work opens new lines of investigation into OPCs and the roles of glia in circuit function. This work also shows a novel regulatory role of OPC-microglia crosstalk in synapse elimination, adding to a growing body of work that demonstrates glia-glia communication modulates brain function (Peferoen et al., 2014; Vainchtein et al., 2018; Vainchtein and Molofsky, 2020). In short, this work is exciting because it advances glial cell biology and generates many new questions to research that could have important translational implications for brain disease. For instance, what is the nature of the signal sent from microglia to OPCs to mediate synapse engulfment and can this signal be targeted therapeutically?
References
Allen, N.J., and D.A. Lyons. 2018. Glia as architects of central nervous system formation and function. Science. 362:181-185.
Barres, B.A. 2008. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 60:430-440.
Barres, B.A., and M.C. Raff. 1993. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 361:258-260.
Bergles, D.E., J.D. Roberts, P. Somogyi, and C.E. Jahr. 2000. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 405:187-191.
Bolton, J.L., A.K. Short, S. Othy, C.L. Kooiker, M. Shao, B.G. Gunn, J. Beck, X. Bai, S.M. Law, J.C. Savage, J.J. Lambert, D. Belelli, M.E. Tremblay, M.D. Cahalan, and T.Z. Baram. 2022. Early stress-induced impaired microglial pruning of excitatory synapses on immature CRH-expressing neurons provokes aberrant adult stress responses. Cell Rep. 38:110600.
Boulanger, J.J., and C. Messier. 2017. Oligodendrocyte progenitor cells are paired with GABA neurons in the mouse dorsal cortex: Unbiased stereological analysis. Neuroscience. 362:127-140.
Cheadle, L., S.A. Rivera, J.S. Phelps, K.A. Ennis, B. Stevens, L.C. Burkly, W.A. Lee, and M.E. Greenberg. 2020. Sensory Experience Engages Microglia to Shape Neural Connectivity through a Non-Phagocytic Mechanism. Neuron. 108:451-468 e459.
Chung, W.S., L.E. Clarke, G.X. Wang, B.K. Stafford, A. Sher, C. Chakraborty, J. Joung, L.C. Foo, A. Thompson, C. Chen, S.J. Smith, and B.A. Barres. 2013. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 504:394-400.
Chung, W.S., P.B. Verghese, C. Chakraborty, J. Joung, B.T. Hyman, J.D. Ulrich, D.M. Holtzman, and B.A. Barres. 2016. Novel allele-dependent role for APOE in controlling the rate of synapse pruning by astrocytes. Proc Natl Acad Sci U S A. 113:10186-10191.
Clayton, B.L.L., and P.J. Tesar. 2021. Oligodendrocyte progenitor cell fate and function in development and disease. Curr Opin Cell Biol. 73:35-40.
Gibson, E.M., D. Purger, C.W. Mount, A.K. Goldstein, G.L. Lin, L.S. Wood, I. Inema, S.E. Miller, G. Bieri, J.B. Zuchero, B.A. Barres, P.J. Woo, H. Vogel, and M. Monje. 2014. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 344:1252304.
Hong, S., V.F. Beja-Glasser, B.M. Nfonoyim, A. Frouin, S. Li, S. Ramakrishnan, K.M. Merry, Q. Shi, A. Rosenthal, B.A. Barres, C.A. Lemere, D.J. Selkoe, and B. Stevens. 2016. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 352:712-716.
Mataga, N., Y. Mizuguchi, and T.K. Hensch. 2004. Experience-dependent pruning of dendritic spines in visual cortex by tissue plasminogen activator. Neuron. 44:1031-1041.
Pease-Raissi, S.E., and J.R. Chan. 2021. Building a (w)rapport between neurons and oligodendroglia: Reciprocal interactions underlying adaptive myelination. Neuron. 109:1258-1273.
Peferoen, L., M. Kipp, P. van der Valk, J.M. van Noort, and S. Amor. 2014. Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology. 141:302-313.
Schafer, D.P., E.K. Lehrman, A.G. Kautzman, R. Koyama, A.R. Mardinly, R. Yamasaki, R.M. Ransohoff, M.E. Greenberg, B.A. Barres, and B. Stevens. 2012. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 74:691-705.
Vainchtein, I.D., G. Chin, F.S. Cho, K.W. Kelley, J.G. Miller, E.C. Chien, S.A. Liddelow, P.T. Nguyen, H. Nakao-Inoue, L.C. Dorman, O. Akil, S. Joshita, B.A. Barres, J.T. Paz, A.B. Molofsky, and A.V. Molofsky. 2018. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science. 359:1269-1273.
Vainchtein, I.D., and A.V. Molofsky. 2020. Astrocytes and Microglia: In Sickness and in Health. Trends Neurosci. 43:144-154.
Yilmaz, M., E. Yalcin, J. Presumey, E. Aw, M. Ma, C.W. Whelan, B. Stevens, S.A. McCarroll, and M.C. Carroll. 2021. Overexpression of schizophrenia susceptibility factor human complement C4A promotes excessive synaptic loss and behavioral changes in mice. Nat Neurosci. 24:214-224.
Questions for the authors:
- Elimination of all microglia is a dramatic manipulation to show they are needed for OPC engulfment. Do you expect that less severe microglial manipulations would affect OPC synaptic engulfment, e.g. inhibiting microglial receptors?
- Do you expect that dependence on microglia for synaptic engulfment by OPCs persists into adulthood?
- There is crosstalk between astrocytes and microglia in regulation of synapse elimination by glia. Do you think astrocytes may play a role in regulating OPC synaptic engulfment too?
- You examined a single cell RNAseq dataset to provide corroborating evidence that OPCs engage in phagocytosis of synaptic inputs. Did that data provide hints about other potential functions of OPCs?
doi: https://doi.org/10.1242/prelights.32166
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the neuroscience category:
PPARδ activation in microglia drives a transcriptional response that primes phagocytic function while countering inflammatory activation
Isabel Paine
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Self-renewal of neuronal mitochondria through asymmetric division
Lorena Olifiers
preLists in the neuroscience category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |











 (No Ratings Yet)
(No Ratings Yet)