Orally Bioavailable Endochin-like Quinolone Carbonate Ester Prodrug Reduces Toxoplasma gondii Brain Cysts
Posted on: 10 April 2020
Preprint posted on 20 March 2020
Article now published in Antimicrobial Agents and Chemotherapy at http://dx.doi.org/10.1128/AAC.00535-20
The quinolone in the ester mask: Doggett et al. adopt a prodrug approach to reduce Toxoplasma gondii brain cysts
Selected by Zhang-He GohCategories: pharmacology and toxicology
Background of preprint
Toxoplasma gondii is a widespread parasite that can be especially dangerous in immunocompromised patients. Unfortunately, the few existing treatments that are effective against T. gondii also have high rates of adverse events that lead to discontinuation in 30% of patients.
About a decade earlier, endochin-like quinolones (ELQ) were found to inhibit apicomplexan pathogens, including T. gondii [1] and Plasmodium falciparum [2]. Doggett et al. had previously identified ELQ-271 and ELQ-316 (Fig. 1) as potential compounds against T. gondii, and specifically highlighted ELQ-316 as a prospective lead due to its “efficacy and specificity for the T. gondii cytochrome b over the human cytochrome b” in their 2012 paper [1]. However, owing to pharmacokinetic issues, the oral dosing of ELQ-316 was not effective.
Figure 1. ELQs discussed in Doggett et al.’s previous work and current preprint

In their preprint, Doggett et al. synthesised and characterised ELQ-334 (Fig. 1), a carbonate ester of ELQ-316, using the carbonate ester-masking approach to increase bioavailability. The preprint authors characterised the pharmacokinetic parameters and efficacy of ELQ-334 against both acute and latent toxoplasmosis.
Key findings of preprint
Doggett et al. administered ELQ-316 and ELQ-334 to mice orally and their pharmacokinetic parameters were characterised in the plasma and brain: cmax, Tmax, AUC0-96, and t1/2 (Table 1).
Table 1. Pharmacokinetic parameters of ELQ-316 from ELQ-316 and ELQ-334.
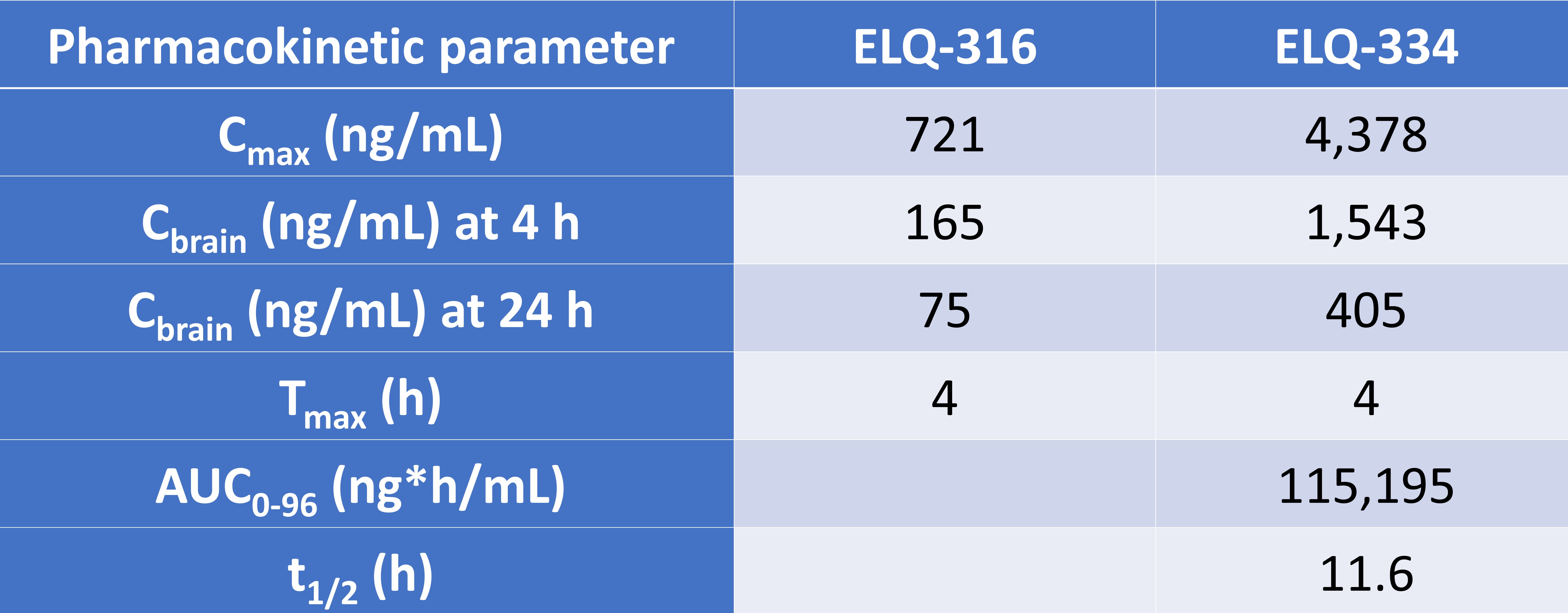
The preprint authors also found that the mean plasma concentration of ELQ-334 did not exceed 238 ng/ml. Taken together, these findings led the preprint authors to conclude that the prodrug ELQ-334 was rapidly converted to the active compound ELQ-316.
By testing the efficacy of ELQ-334 against acute toxoplasmosis in mice, Doggett et al. found that the administration of ELQ-334 significantly boosted survivability of the mice. The authors also tested ELQ-334 in latent T. gondii infection and found that ELQ-334 significantly reduced the number of T. gondii cysts compared to control mice.
What I like about this preprint
I selected this preprint because it illustrates an important tenet that underlies the work done by researchers and healthcare professionals: for a drug to be effective, it needs to reach effective concentrations at the site of action. For decades, the brain has presented a challenge to anyone working in the healthcare sector. Formulators and medicinal chemists try different strategies to help drugs penetrate the Blood-Brain Barrier (BBB); while pharmacists and physicians carefully consider the pharmacokinetics of a drug in relation to the site of infection before prescribing or dispensing it to patients.
Events in recent months have once again underscored the need for research into combating infectious diseases. As the global population—especially the subset residing in the developed world—continues to age, patients are becoming increasingly complex and vulnerable. The twin threats of an ageing population and growing antimicrobial resistance will fuel the urgency for more potent antimicrobials in this decade. At the same time, these antimicrobials also need to be sufficiently selective to inflict minimal side effects on their hosts.
From this perspective, the findings that Doggett et al. have highlighted in this preprint will impact many of these considerations. The first is that ELQ-316 was selected over ELQ-271 for optimisation, owing to ELQ-316’s higher potency and better selectivity [1]. Then there is the challenge to optimise the absorption pharmacokinetics of ELQ-316, which the preprint authors resolved by creating ELQ-334, a prodrug carbonate ester of ELQ-316. Finally, the preprint authors also explored the role that ELQ-334 might play in eradicating latent infections, a consideration that will become increasingly relevant as we further develop healthcare capabilities to extend the human lifespan and support medically vulnerable populations.
Questions for authors
- In the noncompartmental pharmacokinetic analysis of ELQ-316 and ELQ-334, you compared the brain concentrations of ELQ-316 derived from ELQ-316 and ELQ-334. In lines 124 – 125 of the preprint, brain concentrations for ELQ-316 derived from ELQ-316 were reported in ng/g, while in lines 122 – 123 of the preprint, the brain concentrations for ELQ-316 derived from ELQ-334 were reported in ng/ml. In 3B of the preprint, was it assumed that the density of brain tissue is 1 g/mL, so that this would allow us to do a direct comparison between the two results?
- What were the AUC0-96 and the t1/2 of ELQ-316 derived from ELQ-316? Were there significant differences between the t1/2 of ELQ-316 derived from ELQ-316 and the t1/2 of ELQ-316 derived from ELQ-334 ( 3A of the preprint)?
- The hypothesis that the conversion of ELQ-334 to ELQ-316 may “be caused in part by esterases in the gastrointestinal tract” (preprint lines 127 – 129) is an interesting one, given that in preprint lines 72 – 73, the decrease in the bioavailability of ELQs was attributed to the precipitation in the gastrointestinal tract. Therefore, it appears to follow that, were the ester cleavage performed in the gastrointestinal tract, the same problems would arise from an absorption perspective. Would it be possible that it is the ubiquitous body esterases—such as those in the liver—that are instead responsible for the first-pass effect?
References
[1] Doggett JS, Nilsen A, Forquer I, Wegmann KW, Jones-Brando L, Yolken RH, Bordón C, Charman SA, Katneni K, Schultz T, Burrows JN, Hinrichs DJ, Meunier B, Carruthers VB, Riscoe MK, Endochin-like quinolones are highly efficacious against acute and latent experimental toxoplasmosis, Proc Natl Acad Sci U S A 109(39) (2012) 15936-15941.
[2] Winter R, Kelly JX, Smilkstein MJ, Hinrichs D, Koop DR, Riscoe MK, Optimization of endochin-like quinolones for antimalarial activity, Exp Parasitol 127(2) (2011) 545-551.
doi: https://doi.org/10.1242/prelights.18397
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the pharmacology and toxicology category:
Snake venom metalloproteinases are predominantly responsible for the cytotoxic effects of certain African viper venoms
Daniel Osorno Valencia
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
In vitro pharmacokinetics and pharmacodynamics of the diarylquinoline TBAJ-587 and its metabolites against Mycobacterium tuberculosis
Zhang-He Goh
preLists in the pharmacology and toxicology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
COVID-19 / SARS-CoV-2 preprints
List of important preprints dealing with the ongoing coronavirus outbreak. See http://covidpreprints.com for additional resources and timeline, and https://connect.biorxiv.org/relate/content/181 for full list of bioRxiv and medRxiv preprints on this topic
| List by | Dey Lab, Zhang-He Goh |
1
Drug use in special populations
Any drugs that are being used in special populations: Patients with liver and kidney failure, in paediatrics, in geriatrics, and in pregnant or lactating patients. Includes the discovery of factors that could potentially affect drug use in these special populations.
| List by | Zhang-He Goh |
Toxicology of toxicants, existing therapeutics, and investigational drugs
Preprints that describe the toxicology of environmental pollutants and existing and upcoming drugs. Includes both toxicokinetics and toxicodynamics, as well as technological improvements that will help in the characterisation of this field.
| List by | Zhang-He Goh |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |
Anticancer agents: Discovery and clinical use
Preprints that describe the discovery of anticancer agents and their clinical use. Includes both small molecules and macromolecules like biologics.
| List by | Zhang-He Goh |
Advances in Drug Delivery
Advances in formulation technology or targeted delivery methods that describe or develop the distribution of small molecules or large macromolecules to specific parts of the body.
| List by | Zhang-He Goh |











 (No Ratings Yet)
(No Ratings Yet)