Pharmacological Insights into Safety and Efficacy Determinants for the Development of Adenosine Receptor Biased Agonists in the Treatment of Heart Failure
Posted on: 8 September 2020
Preprint posted on 23 July 2020
Article now published in Frontiers in Pharmacology at http://dx.doi.org/10.3389/fphar.2021.628060
My heart will go on: researchers characterise the pharmacology of the adenosine receptor amidst heart failure drug discovery efforts
Selected by Zhang-He GohCategories: pharmacology and toxicology
Background of preprint: the spaces between ARs
Adenosine receptors (ARs) are key mediators in cardiovascular function. They control heart rate and conduction [1,2], autonomic control and vasoregulation [3,4], perfusion, and growth and remodelling. Their agonism can offer protection from injury [5]: A1R agonists reduce the neurohormonal [6] and inflammation [7,8] responses [9-11]; while A2BR agonists promote cardiac fibroblast proliferation and collagen synthesis [12-16].
The strategy of targeting ARs to mitigate heart failure is currently complicated by ARs’ systemic expression and their multiple roles in the modulation of other cardiac [3,4] and renal [17,18] processes. Despite these difficulties, some selectivity has since been achieved by exploiting ARs’ ability to exhibit biased agonism with VCP746 and its analogues [19,20]. In fact, Bayer’s capadenoson [21,22] and neladenoson [23-27] progressed into preclinical models and clinical trials, suggesting that such a strategy may indeed be viable. Therefore, to enhance our understanding of these characteristics, Rueda et al. characterised and compared the preclinical pharmacology of VCP746, capadenoson, neladenoson, and the pan-AR agonist NECA (Fig. 1).
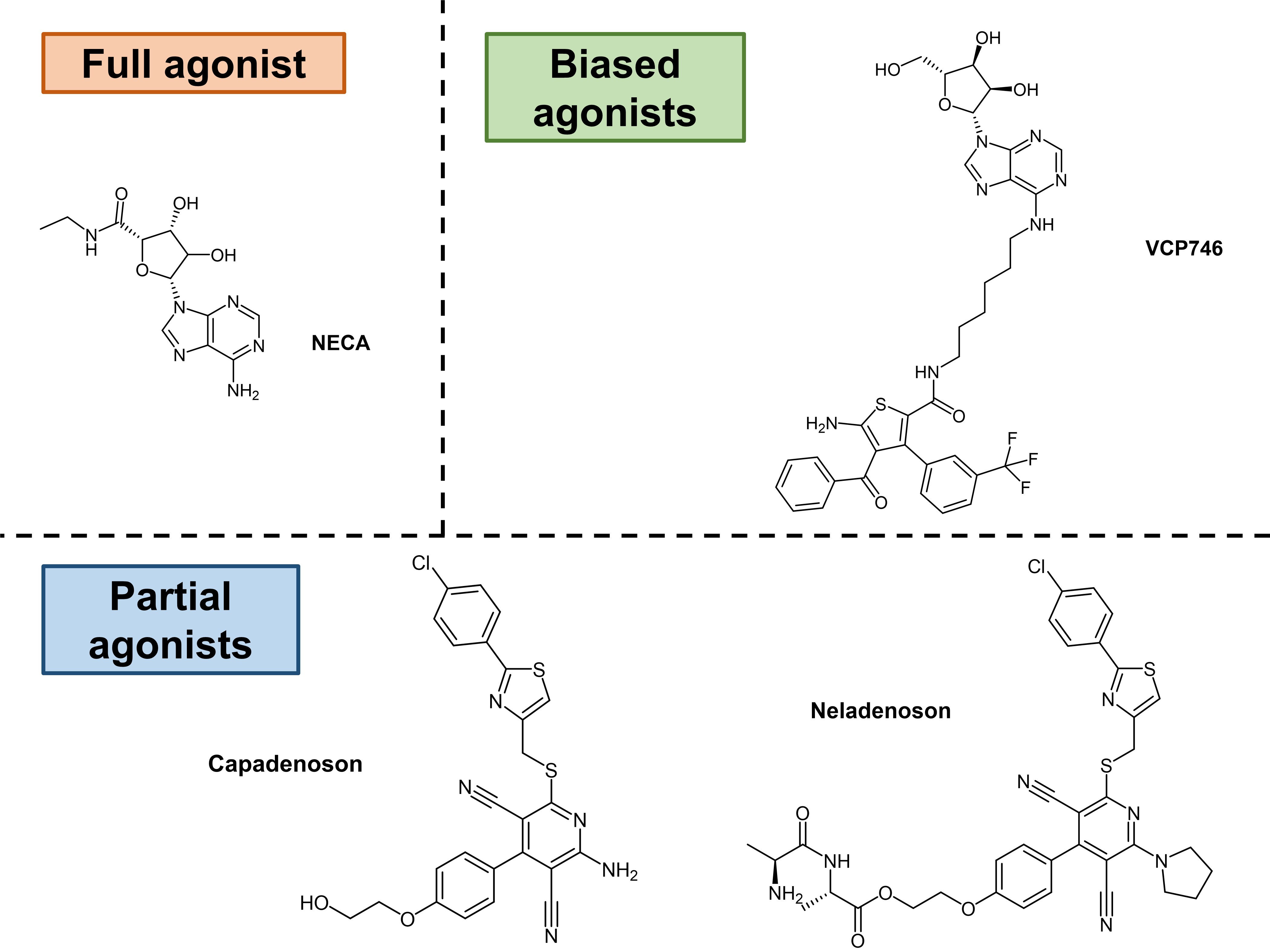
Figure 1. Agonists investigated by Rueda et al. in their preprint.
Key findings of preprint: opening the door to the heart
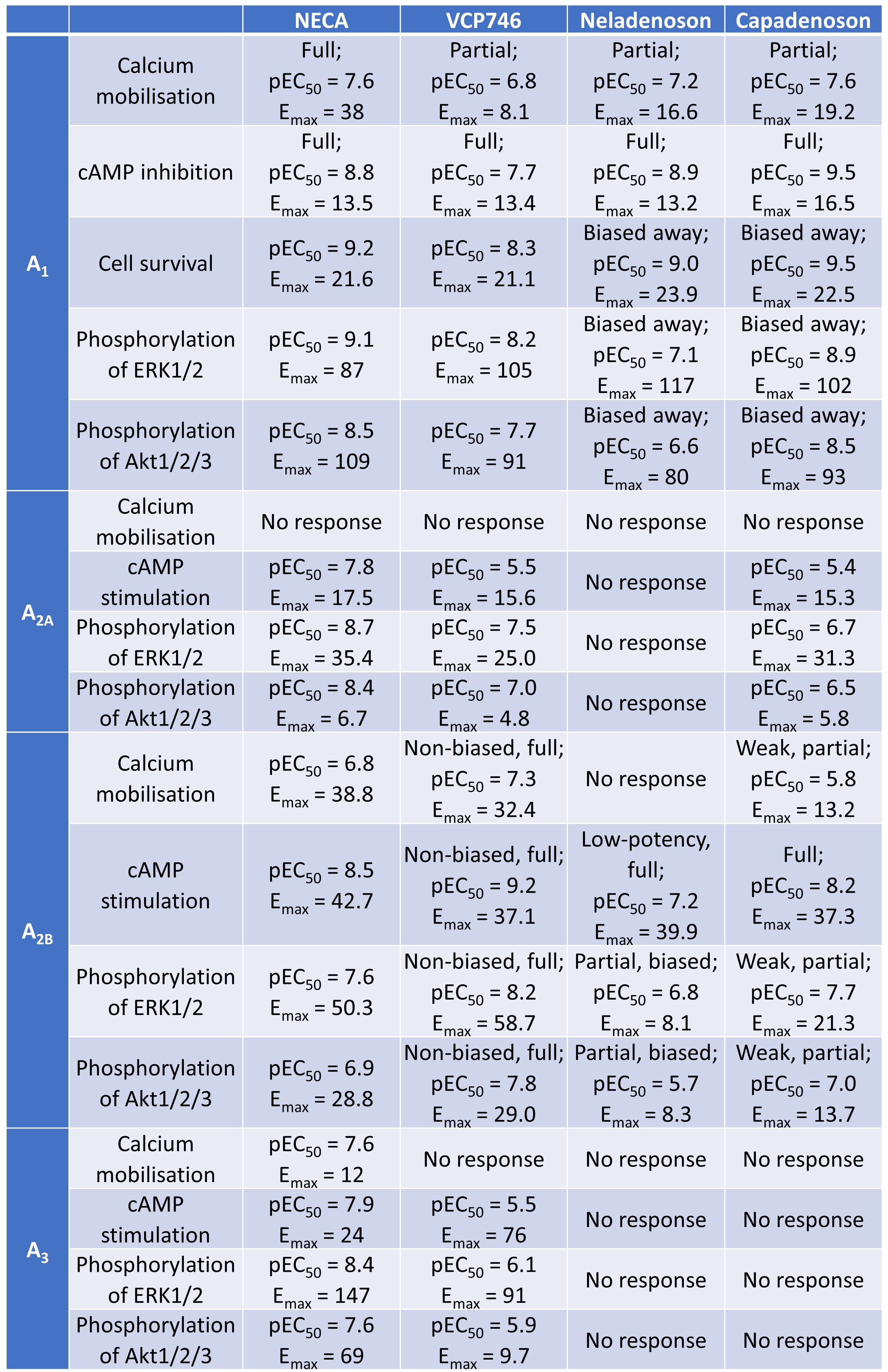
Rueda et al. first characterised the differential activity of VCP746, neladenoson, and capadenoson at A1 and A2B receptors, comparing their pharmacological profiles to the full agonist NECA. They made several key findings (Table 1):
- VCP746 exhibited a high potency at A2BR, weak activity at A2AR, and minimal activity at A3R
- Capadenoson’s selectivity for ARs ran in the following descending order: A1R > A2BR > A2AR >> A3R
- Neladenoson was active at A2BR, but did not exhibit appreciable activity at A2AR or A3R
- Neladenoson was a selective A1R biased agonist, with biased, weak agonism at the A2BR subtype
- VCP746 was a biased A1R agonist and potent unbiased agonist at A2B
Rueda et al. then assessed the physiological effects of VCP746 and neladenoson. The authors first found that these two agents are anti-hypertrophic in cardiomyoctes—an effect associated with A1R inhibition [9], though these inhibitory effects were not caused by a reduction in cell viability. VCP746 and neladenoson were also anti-fibrotic in cardiac fibroblasts via their A2BR agonism.
Next, the authors characterised the in vitro, ex vivo, and in vivo effects of VCP746 and neladonoson on heart rate. In primary rat neonatal ventricular cardiomyocytes (NVCM), neladoson, but not VCP746, induced a slow heart rate through A1R-agonist-induced negative chronotropy. In ex vivo rat-isolated right atria, both neladenoson and VCP746 had minimal effects when compared to NECA. In vivo, neladenoson decreased heart rate in rats, while VCP746 increased it.
Table 1. Activities of VCP746, neladenoson, and capadenoson at various ARs.
Because renal disease often manifests in patients with heart failure, Rueda et al. next investigated the roles that ARs play in renal haemodynamics. The authors showed that A2BR-mediated vasodilation could be induced by VCP746 and neladenoson, but not A1R-mediated vasoconstriction; they ascribed the latter finding to the biased agonism of VCP746 and neladenoson. In the same vein (pun intended), the authors found that VCP746 and neladenoson also did not induce A2AR-mediated rat thoracic aorta relaxation in vivo.
What I like about this preprint: ARs—a lifetime of research
I picked this preprint because it answers an interesting research question. As Rueda et al. point out, pharmacologists have been targeting ARs (especially A1R) for over two decades. Yet, efforts in creating a selective A1R agonist have been stymied by adverse effects arising from the difficulty of selective A1R agonism—the widespread distribution of A1R and its myriad physiological effects make such approaches too risky. Two main strategies have since been developed to target this problem: partial agonism, as seen in Bayer’s capadenoson and neladenoson; and biased A1R agonism, which elicit different effects despite targeting A1R.
In their preprint, Rueda et al. seek to clarify the pharmacology of targeting ARs. The authors’ in vitro, ex vivo, and in vivo work suggests that AR full and partial agonists indeed exhibit different pharmacodynamics and elicit different pharmacological responses at different sites. The authors’ findings gathered from a range of laboratory models allowed them to compare these differential pharmacological responses (Table 1). These findings in turn may shed some light on the finding that capadenoson and neladenoson did not appear to affect heart rate in clinical trials [25,26].
Future work: research into ARs and the heart will go on and on
Given the vast complexity in AR pharmacology, future work will be necessary to better understand the pharmacology of A1R agonism. A few key questions remain.
The authors, for one, ask whether A1R agonists with a cAMP-calcium bias but without the bias away from cAMP signalling is possible. A medicinal chemistry campaign involving the development of a structural understanding and the generation of the structure-activity relationship will be needed to answer this question.
Moreover, the lack of clinical translation should also be taken into consideration vis-à-vis the authors’ findings in various models. Traditionally, preclinical models involve the use of rodents (e.g. mice and rats) and non-rodents (e.g. dogs and monkeys) in testing, in addition to various in vitro tests. But to what extent do these reflect the pharmacodynamics of these agents in the human body?
One last major consideration involves the pharmacokinetics of potential AR agonists. Even if a selective and biased A1R agonist can be developed, what will the pharmacokinetic (PK)-pharmacodynamic (PD) relationship be? The multitude of factors at play, such as the partial agonist properties of these agents, may further complicate the PK-PD relationship. This in turn may have implications for clinical dosing.
All in all, the findings by Rueda et al. point to the difficulties in manipulating AR pharmacology. Until the roles of AR in cardiac function are more clearly identified, research into the relationship between AR agonism and its cardiac effects will go on.
Open questions
- It is interesting that selective A1R targeting can have so many physiological effects, giving rise to the phenomenon of partial agonism. Has the molecular basis for this phenomenon been established? How do you think this will be further developed moving forward?
References
[1] Belardinelli L, Shryock JC, Song Y, Wang D, Srinivas M, Ionic basis of the electrophysiological actions of adenosine on cardiomyocytes, Faseb j 9(5) (1995) 359-365.
[2] Yang JN, Tiselius C, Daré E, Johansson B, Valen G, Fredholm BB, Sex differences in mouse heart rate and body temperature and in their regulation by adenosine A1 receptors, Acta Physiol (Oxf) 190(1) (2007) 63-75.
[3] Kemp BK, Cocks TM, Adenosine mediates relaxation of human small resistance-like coronary arteries via A2B receptors, Br J Pharmacol 126(8) (1999) 1796-1800.
[4] Sato A, Terata K, Miura H, Toyama K, Loberiza FR, Jr., Hatoum OA, Saito T, Sakuma I, Gutterman DD, Mechanism of vasodilation to adenosine in coronary arterioles from patients with heart disease, Am J Physiol Heart Circ Physiol 288(4) (2005) H1633-1640.
[5] Headrick JP, Ashton KJ, Rose’meyer RB, Peart JN, Cardiovascular adenosine receptors: expression, actions and interactions, Pharmacol Ther 140(1) (2013) 92-111.
[6] Frey N, Olson EN, Cardiac hypertrophy: the good, the bad, and the ugly, Annu Rev Physiol 65 (2003) 45-79.
[7] Kuusisto J, Kärjä V, Sipola P, Kholová I, Peuhkurinen K, Jääskeläinen P, Naukkarinen A, Ylä-Herttuala S, Punnonen K, Laakso M, Low-grade inflammation and the phenotypic expression of myocardial fibrosis in hypertrophic cardiomyopathy, Heart 98(13) (2012) 1007-1013.
[8] Erten Y, Tulmac M, Derici U, Pasaoglu H, Altok Reis K, Bali M, Arinsoy T, Cengel A, Sindel S, An association between inflammatory state and left ventricular hypertrophy in hemodialysis patients, Ren Fail 27(5) (2005) 581-589.
[9] Chuo CH, Devine SM, Scammells PJ, Krum H, Christopoulos A, May LT, White PJ, Wang BH, VCP746, a novel A1 adenosine receptor biased agonist, reduces hypertrophy in a rat neonatal cardiac myocyte model, Clin Exp Pharmacol Physiol 43(10) (2016) 976-982.
[10] Puhl SL, Kazakov A, Müller A, Fries P, Wagner DR, Böhm M, Maack C, Devaux Y, Adenosine A1 receptor activation attenuates cardiac hypertrophy and fibrosis in response to α1 -adrenoceptor stimulation in vivo, Br J Pharmacol 173(1) (2016) 88-102.
[11] Liao Y, Lin L, Lu D, Fu Y, Bin J, Xu D, Kitakaze M, Activation of adenosine A1 receptor attenuates tumor necrosis factor-α induced hypertrophy of cardiomyocytes, Biomedicine & Pharmacotherapy 65(7) (2011) 491-495.
[12] Epperson SA, Brunton LL, Ramirez-Sanchez I, Villarreal F, Adenosine receptors and second messenger signaling pathways in rat cardiac fibroblasts, Am J Physiol Cell Physiol 296(5) (2009) C1171-1177.
[13] Chen Y, Epperson S, Makhsudova L, Ito B, Suarez J, Dillmann W, Villarreal F, Functional effects of enhancing or silencing adenosine A2b receptors in cardiac fibroblasts, Am J Physiol Heart Circ Physiol 287(6) (2004) H2478-2486.
[14] Dubey RK, Gillespie DG, Mi Z, Jackson EK, Exogenous and Endogenous Adenosine Inhibits Fetal Calf Serum–Induced Growth of Rat Cardiac Fibroblasts, Circulation 96(8) (1997) 2656-2666.
[15] Dubey RK, Gillespie DG, Zacharia LC, Mi Z, Jackson EK, A<sub>2B</sub> Receptors Mediate the Antimitogenic Effects of Adenosine in Cardiac Fibroblasts, Hypertension 37(2) (2001) 716-721.
[16] Dubey RK, Gillespie DG, Jackson EK, Adenosine inhibits collagen and protein synthesis in cardiac fibroblasts: role of A2B receptors, Hypertension 31(4) (1998) 943-948.
[17] Damman K, Testani JM, The kidney in heart failure: an update, European Heart Journal 36(23) (2015) 1437-1444.
[18] Vallon V, Miracle C, Thomson S, Adenosine and kidney function: potential implications in patients with heart failure, Eur J Heart Fail 10(2) (2008) 176-187.
[19] Baltos JA, Gregory KJ, White PJ, Sexton PM, Christopoulos A, May LT, Quantification of adenosine A(1) receptor biased agonism: Implications for drug discovery, Biochem Pharmacol 99 (2016) 101-112.
[20] Valant C, May LT, Aurelio L, Chuo CH, White PJ, Baltos JA, Sexton PM, Scammells PJ, Christopoulos A, Separation of on-target efficacy from adverse effects through rational design of a bitopic adenosine receptor agonist, Proc Natl Acad Sci U S A 111(12) (2014) 4614-4619.
[21] Albrecht-Küpper BE, Leineweber K, Nell PG, Partial adenosine A1 receptor agonists for cardiovascular therapies, Purinergic Signal 8(Suppl 1) (2012) 91-99.
[22] Sabbah HN, Gupta RC, Kohli S, Wang M, Rastogi S, Zhang K, Zimmermann K, Diedrichs N, Albrecht-Küpper BE, Chronic therapy with a partial adenosine A1-receptor agonist improves left ventricular function and remodeling in dogs with advanced heart failure, Circ Heart Fail 6(3) (2013) 563-571.
[23] Meibom D, Albrecht-Küpper B, Diedrichs N, Hübsch W, Kast R, Krämer T, Krenz U, Lerchen HG, Mittendorf J, Nell PG, Süssmeier F, Vakalopoulos A, Zimmermann K, Neladenoson Bialanate Hydrochloride: A Prodrug of a Partial Adenosine A(1) Receptor Agonist for the Chronic Treatment of Heart Diseases, ChemMedChem 12(10) (2017) 728-737.
[24] Voors AA, Düngen HD, Senni M, Nodari S, Agostoni P, Ponikowski P, Bax JJ, Butler J, Kim RJ, Dorhout B, Dinh W, Gheorghiade M, Safety and Tolerability of Neladenoson Bialanate, a Novel Oral Partial Adenosine A1 Receptor Agonist, in Patients With Chronic Heart Failure, J Clin Pharmacol 57(4) (2017) 440-451.
[25] Voors AA, Bax JJ, Hernandez AF, Wirtz AB, Pap AF, Ferreira AC, Senni M, van der Laan M, Butler J, Safety and efficacy of the partial adenosine A1 receptor agonist neladenoson bialanate in patients with chronic heart failure with reduced ejection fraction: a phase IIb, randomized, double-blind, placebo-controlled trial, Eur J Heart Fail 21(11) (2019) 1426-1433.
[26] Shah SJ, Voors AA, McMurray JJV, Kitzman DW, Viethen T, Bomfim Wirtz A, Huang E, Pap AF, Solomon SD, Effect of Neladenoson Bialanate on Exercise Capacity Among Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial, Jama 321(21) (2019) 2101-2112.
[27] Bertero E, Maack C, The Partial AdeNosine A1 receptor agonist in patients with Chronic Heart failure and preserved Ejection fraction (PANACHE) trial, Cardiovasc Res 115(8) (2019) e71-e73.
doi: https://doi.org/10.1242/prelights.24510
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the pharmacology and toxicology category:
G6b-B antibody-based cis-acting platelet receptor inhibitors (CAPRIs) as a new family of anti-thrombotic therapeutics
Simon Cleary
Pervasive sublethal effects of agrochemicals as contributing factors to insect decline
Roberto Amadio
Mixed Alkyl/Aryl Phosphonates Identify Metabolic Serine Hydrolases as Antimalarial Targets
Zhang-He Goh
preLists in the pharmacology and toxicology category:
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
COVID-19 / SARS-CoV-2 preprints
List of important preprints dealing with the ongoing coronavirus outbreak. See http://covidpreprints.com for additional resources and timeline, and https://connect.biorxiv.org/relate/content/181 for full list of bioRxiv and medRxiv preprints on this topic
| List by | Dey Lab, Zhang-He Goh |
1
Drug use in special populations
Any drugs that are being used in special populations: Patients with liver and kidney failure, in paediatrics, in geriatrics, and in pregnant or lactating patients. Includes the discovery of factors that could potentially affect drug use in these special populations.
| List by | Zhang-He Goh |
Toxicology of toxicants, existing therapeutics, and investigational drugs
Preprints that describe the toxicology of environmental pollutants and existing and upcoming drugs. Includes both toxicokinetics and toxicodynamics, as well as technological improvements that will help in the characterisation of this field.
| List by | Zhang-He Goh |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |
Anticancer agents: Discovery and clinical use
Preprints that describe the discovery of anticancer agents and their clinical use. Includes both small molecules and macromolecules like biologics.
| List by | Zhang-He Goh |
Advances in Drug Delivery
Advances in formulation technology or targeted delivery methods that describe or develop the distribution of small molecules or large macromolecules to specific parts of the body.
| List by | Zhang-He Goh |











 (No Ratings Yet)
(No Ratings Yet)