Regulation of modulatory cell activity across olfactory structures in Drosophila melanogaster
Posted on: 4 February 2019
Preprint posted on 16 January 2019
Multifaceted cells in the brain – Upon receiving a stimulus, a huge serotonergic neuron can simultaneously provide different outputs at its different target-sites.
Selected by Rudra Nayan DasCategories: animal behavior and cognition, neuroscience, physiology
Background
Different sensory systems, by extracting specific forms of energies reflected or generated by the objects around, allow communication of the external world with the internal body states (Nelson & MacIver, 2006). The sensory systems perform multiple levels of processing on the incoming information in order to extract what is relevant for the organism. Neuromodulators play an important role in sensory processing and are vital for understanding the context-dependent regulation of sensory information. Serotonin is one such neuromodulator, whose influence on local circuits is often complex and multifaceted.
The olfactory sensory system of the fruit fly, Drosophila melanogaster, is one of the well-studied neural circuits. The basic architecture of the olfactory system in insects shares a high degree of similarity with that of its mammalian counterpart. The availability of numerous transgenic tools allows the identification and manipulation of a subset of neurons, which make the Drosophila olfactory system an attractive model to answer questions related to sensory regulation. Furthermore, an understanding of neuronal connectivity has been greatly accelerated through the release of freely available, synaptic resolution electron microscopy data from the entire adult Drosophila brain (Zheng et al., 2018).
In the fruit fly brain, the serotonergic input in the primary olfactory centre (antennal lobe) comes only from a pair of neurons called the contralaterally projecting, serotonin-immunoreactive deutocerebral neurons (CSDn). Interestingly, each of these neurons send projections to second order processing centres (lateral horn and mushroom body) and to third order processing centre – the superior lateral protocerebrum (Coates et al., 2017). Thus, each CSDn represents a widely projecting modulatory neuron innervating different areas of the brain. In this work, the authors compared odor-evoked activity between the CSDn neurites in the antennal lobe and the lateral horn, which yielded surprising insights.
Important results
Zhang et al. utilized fluorescent microscopic techniques for monitoring neural activity in the CSDn. The authors found that all the tested odors (with the exception of Ammonia) inhibit activity in the CSDn neurites of the antennal lobe (AL) (as also shown previously by the same group in Zhang & Gaudry, 2016). Although odors inhibited CSDn at the level of AL, the higher level arbors in the lateral horn (LH) showed odor-induced excitation. Thus, for the same stimulus, different neurites of the CSDn show simultaneous inhibition and excitation. Moreover, while all the AL-neurites of CSDn seem to be uniformly inhibited, the LH-neurites showed odor-specific spatially segregated excitation pattern.
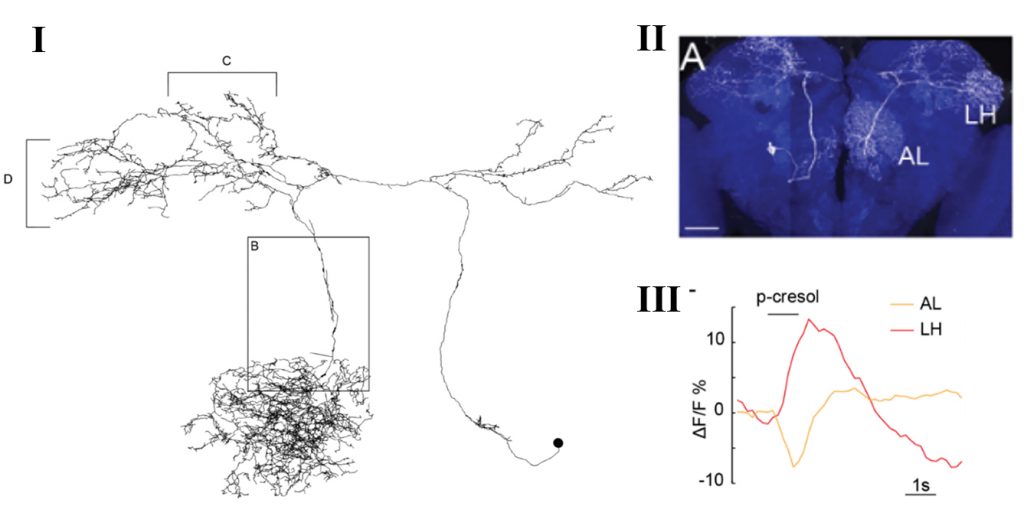
To determine whether the AL and LH compartment’s physiology is interlinked or independently regulated, they performed simulation experiments by constructing a computer model of CSDn. The model utilized EM reconstructions from the whole fly brain EM dataset (Zheng et al., 2018), and the parameters measured from in vivo electrophysiological recordings of the CSDn. Through simulations, the authors determined that the best fit model predicts preferential propagation of voltage from the AL to the LH. This prediction found support in their in vivo experiment where 2-photon laser-mediated transection of the CSDn connection between the LH and AL showed increased odor-evoked excitation in the now isolated LH neurites. Thus, although spatially segregated neurites are activated or inhibited through their local inputs, the local voltage changes do have a role in shaping the final output of distant neurites. Such influence on distant neurites are influenced by the geometry of the neuron, that favours one direction of voltage propagation over the other.
Why I chose this preprint
This work, along with their previous work (Zhang & Gaudry, 2016), that demonstrates serotonergic CSDn to be co-expressing the excitatory neurotransmitter acetylcholine, brings forward a very multifaceted neuron, that seems to be equipped with different modes of target regulation. Modulatory neurotransmitters often have opposite or context-dependent effects on different local circuits of the brain. This work takes it one notch further by showing how a single modulatory neuron can have spatially segregated differential activity. This work is likely to accelerate search for similar modes of local circuit regulation in other systems, although identification and manipulation of identified neurons, across different animals, is highly challenging in vertebrates.
Future Directions
With an enhanced understanding of CSDn physiology, the next challenge will be to establish a concrete causal link between CSDn activity and the animal’s behavioural states. Serotonin has often been known to regulate complex behaviours, and as CSDn has input sites in multiple areas of the brain, it is likely that its physiology will be influenced by other factors. This can provide contextual information that may influence olfactory processing through CSDn.
Additional factors are also likely to add to the complexity of serotonin regulation in the olfactory system. Drosophila has five serotonin receptors that, if expressed differentially in the post-synaptic partners of CSDn, can exert differential responses through CSDn-mediated serotonin. Experience-dependent changes might also alter synaptic strength or numbers of CSDn neurites, that can induce differences in CSDn-mediated odor processing.
Questions to the authors
- Earlier work by the same authors (Zhang & Gaudry, 2016) demonstrated CSDn-mediated serotonin to have inhibitory effects on AL neurons. Can the authors comment on the likely effects of CSDn stimulation in LH? Do CSDn terminals in LH also co-express serotonin and acetylcholine?
- Ammonia was the only odor that induced excitation in both the AL and LH neurites. Is ammonia-sensing circuitry known to be different than others? Can the authors provide some insights into this exception?
- In the two-photon laser transection experiment, judging by the size of the cavitation bubble (Fig. 4 G), it is very likely that other neurons that connect AL and LH are also getting severed. In that scenario, the changes observed maybe a consequence of the non-CSDn transection, which might not be accounted for by the contralateral transection control. In the absence of controls that injures the neighbouring areas (while keeping the CSDn intact), can the authors discard that possibility?
References
Coates, K. E., Majot, A. T., Zhang, X., Michael, C. T., Spitzer, S. L., Gaudry, Q., & Dacks, A. M. (2017). Identified Serotonergic Modulatory Neurons Have Heterogeneous Synaptic Connectivity within the Olfactory System of Drosophila. The Journal of Neuroscience.
Nelson, M. E., & MacIver, M. A. (2006). Sensory acquisition in active sensing systems. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology.
Zhang, X., & Gaudry, Q. (2016). Functional integration of a serotonergic neuron in the drosophila antennal lobe. ELife.
Zheng, Z., Lauritzen, J. S., Perlman, E., Robinson, C. G., Nichols, M., Milkie, D., … Bock, D. D. (2018). A Complete Electron Microscopy Volume of the Brain of Adult Drosophila melanogaster. Cell.
doi: https://doi.org/10.1242/prelights.8239
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the animal behavior and cognition category:
Cannibalism as a mechanism to offset reproductive costs in three-spined sticklebacks
Tina Nguyen
Morphological variations in external genitalia do not explain the interspecific reproductive isolation in Nasonia species complex (Hymenoptera: Pteromalidae)
Stefan Friedrich Wirth
Trade-offs between surviving and thriving: A careful balance of physiological limitations and reproductive effort under thermal stress
Tshepiso Majelantle
Also in the neuroscience category:
Electrophysiological correlates of conscious experiences during sleep: Lucid dreams, sleep paralysis, out-of-body experiences, and false awakenings
uMontreal Neuro preLighters et al.
PPARδ activation in microglia drives a transcriptional response that primes phagocytic function while countering inflammatory activation
Isabel Paine
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Also in the physiology category:
Resilience to cardiac aging in Greenland shark Somniosus microcephalus
Theodora Stougiannou
Trade-offs between surviving and thriving: A careful balance of physiological limitations and reproductive effort under thermal stress
Tshepiso Majelantle
Imaging cellular activity simultaneously across all organs of a vertebrate reveals body-wide circuits
Muhammed Sinan Malik
preLists in the animal behavior and cognition category:
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Bats
A list of preprints dealing with the ecology, evolution and behavior of bats
| List by | Baheerathan Murugavel |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Also in the neuroscience category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |
Also in the physiology category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |











 (No Ratings Yet)
(No Ratings Yet)