Systematic analysis of protein targets associated with adverse events of drugs from clinical trials and post-marketing reports
Posted on: 13 July 2020
Preprint posted on 13 June 2020
Article now published in Chemical Research in Toxicology at http://dx.doi.org/10.1021/acs.chemrestox.0c00294
Toward in vivo-relevant hERG safety assessment and mitigation strategies based on relationships between non-equilibrium blocker binding, three-dimensional channel-blocker interactions, dynamic occupancy, dynamic exposure, and cellular arrhythmia
Posted on:
Preprint posted on 9 June 2020
Article now published in PLOS ONE at http://dx.doi.org/10.1371/journal.pone.0234946
Mitigating the “harm” in “pharm”: strategies to predict, identify, and mitigate drug adverse reactions
Selected by Zhang-He GohCategories: pharmacology and toxicology
Background of preprints
Adverse drug reactions (ADRs) are a major source of economic and clinical burden worldwide. Not only do they cost billions of dollars in the USA alone, but they also weigh heavily on healthcare systems and can account for up to 30% of hospital admissions in the USA and Canada [1]. Although the majority of ADRs are dose-dependent and predictable [2], quantitative information on drug safety has not been organised systematically [3,4]. As such, the prediction of in vivo toxicities in humans remains a challenge.
One common target associated with toxicity is the human ether-a-go-go-related (hERG) voltage-gated cardiac ion channel; drug candidates are screened for the risk of hERG-associated cardiac toxicity before advancing them to clinical trials. However, current late-stage preclinical hERG safety assessments test for cardiac toxicity in dogs or primates at levels far exceeding human therapeutic free plasma cmax (TFPCmax). Yet, the hERG therapeutic index in humans cannot be predicted reliably in the absence of human pharmacokinetic data, leading to a Catch-22 in which safety indices are instead generated based on off-target potency/exposure relationships. Consequently, predictions generally remain inaccurate, despite the significant amounts of time and effort spent on the mitigation of cardiac toxicity.
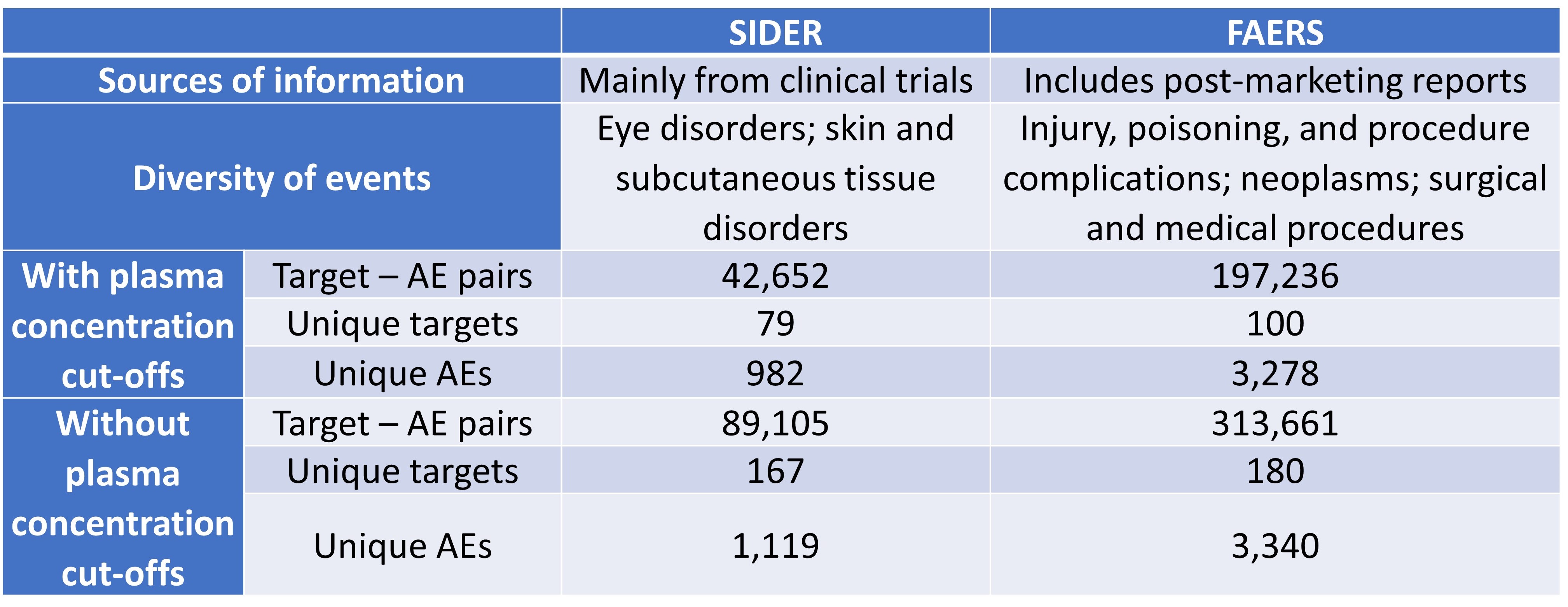
In this preLight, we look at two preprints by Smit et al. and Wan et al. in mitigating these issues associated with toxicities. In the first preprint, Smit et al. target the problems associated with the prediction of pharmaceutical toxicities by analysing data amassed from Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS), Side Effect Resource (SIDER), and in vitro bioactivity data from the CheMBL database (supplemented with ligand-based target predictions). The key difference between FAERS and SIDER lies in their information sources (Table 1): SIDER comprises data from clinical trials, while FAERS also contains post-marketing reports of adverse events (AEs) submitted by healthcare practitioners, consumers, and drug manufacturers. AEs are any adverse episodes following the consumption of a drug; ADRs are AEs for which causality with the agent has been established.
In the second preprint, Wan et al. used biodynamics principles to understand the structural basis of hERG blockade and make hERG safety assessment more relevant in vivo. Specifically, the authors investigated the hERG blockade by trappable and non-trappable compounds, respectively defined as compounds that are trapped within closed hERG channels and compounds that are expelled during closing [5].
Key findings of preprints
(A) Smits et al.
The authors first compared the FAERS and SIDER datasets (Table 1), then evaluated each dataset by comparing them to three previously reported safety target panels [4,6,7], and found that using plasma concentrations resulted in fewer false-positive signals compared to using a constant cut-off. Despite the greater positive predictive values (PPVs) for analyses using plasma concentrations cut-offs, Smit et al. found that the predictive power of the analyses was limited by data availability. Interestingly, the authors observed lower PPVs for FAERS compared to SIDER; they attributed this difference to the greater noise and uncertainty associated with FAERS. Further calculations of value-added PPVs also indicated that target bioactivities provided additional information, and that the PPVs were not solely driven by the prevalence of AEs.
Table 1. Characteristics of FAERS and SIDER datasets.
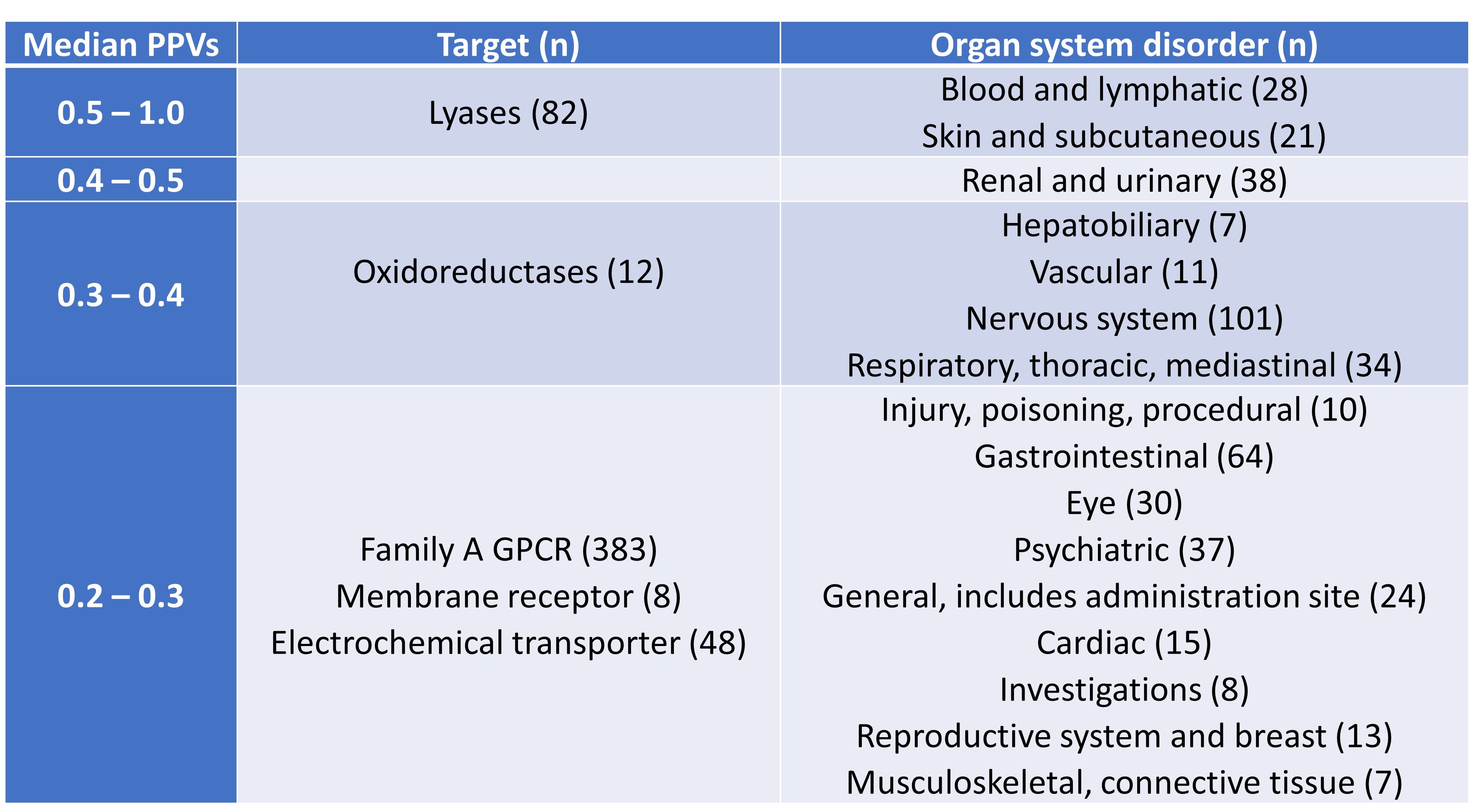
Smit et al. then conducted analyses of PPVs by target and organ class (Table 2). This showed that PPVs were generally high for lyases, while no clear pattern emerged for the analysis of PPVs by organ class. The authors also found a necessary trade-off between the PPVs and detection of AE-associated drugs, which led them to conclude that no single bioactivity is a strong indicator of AEs in both the FAERS and SIDER datasets.
Table 2. PPVs by target and organ class, listed in descending order of PPVs.
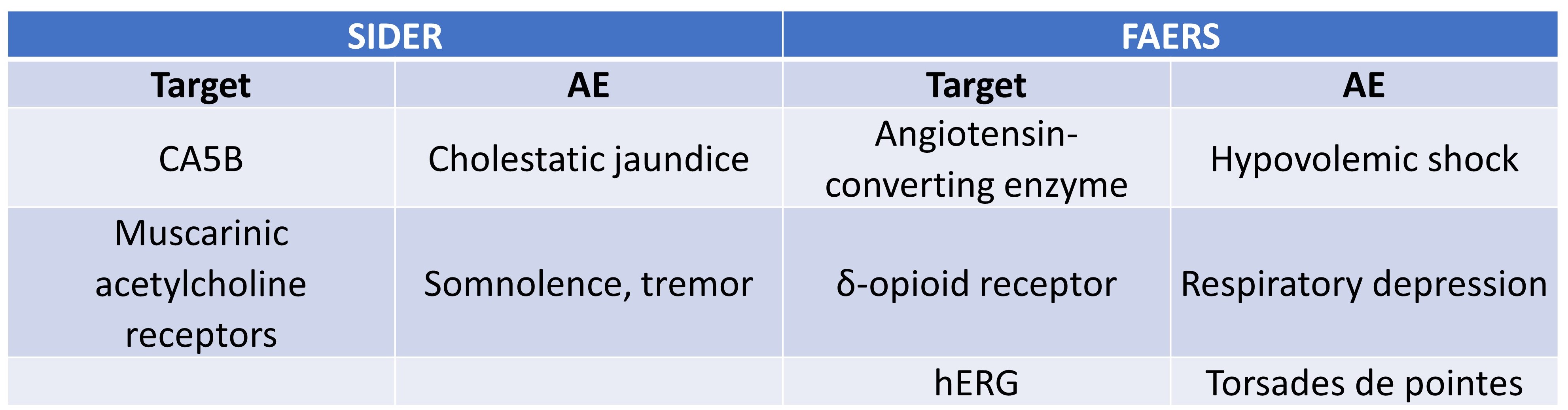
Next, Smit et al. identified a few significant target-AE associations in their study (Table 3), though these interactions were not uniformly distributed across system organ classes. For example, metabolism and nutrition disorders, as well as nervous system disorders, were more frequently associated with certain pharmacological targets. Moreover, only a small fraction of unique AEs was associated with target activity in safety pharmacology screens. It is to this second issue to which we now turn.
Table 3. Significant target-AE pairs from FAERS and SIDER datasets.
(B) Wan et al.
The authors’ investigations revealed that blocker binding is mainly determined by four factors: (1) steric shape and size complementarity between blockers and the pore-binding region; (2) blocker de-solvation rates; (3) channel re-solvation rates; and (4) blocker basicity, which in turn determines electrostatic interactions (Fig. 1).
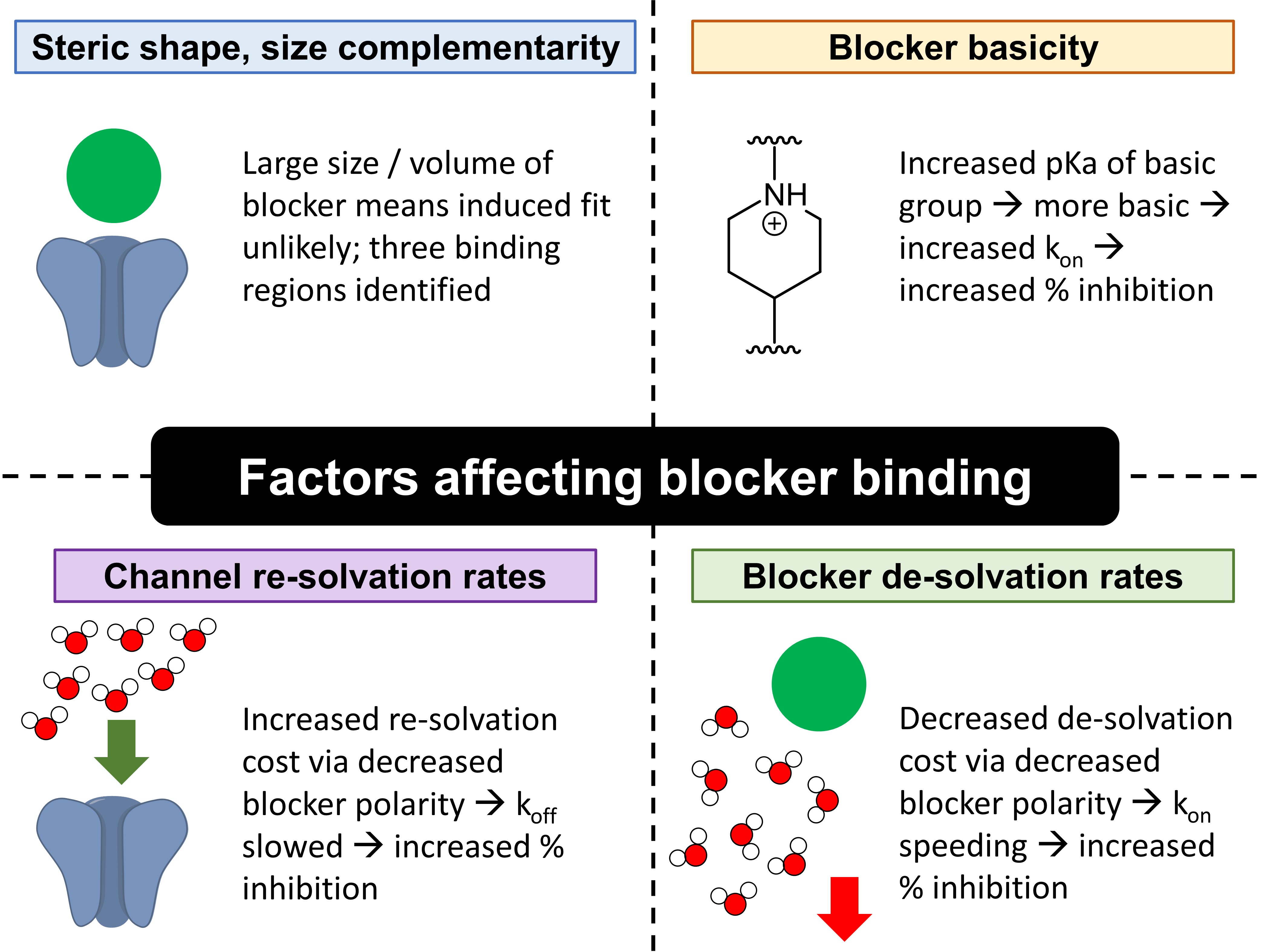
Figure 1. Factors determining blocker binding.
By performing computational analyses (preprint Figure 4), Wan et al. identified structure-kinetics relationships, the kinetic corollary of structure-activity relationships (SAR) (Fig. 1), as well as structure-trappability relationships. Specifically, they showed that trappable and non-trappable analogues exhibit planar and non-planar conformations respectively (preprint Figure 7); the non-planar conformations of non-trappable blockers tend to sterically clash with a region of the pore that may constrict in the closed-channel state. In light of these findings, Wan et al. proposed a set of recommendations to overcome problems traditionally associated with the assessment of hERG blockade (Fig. 2).
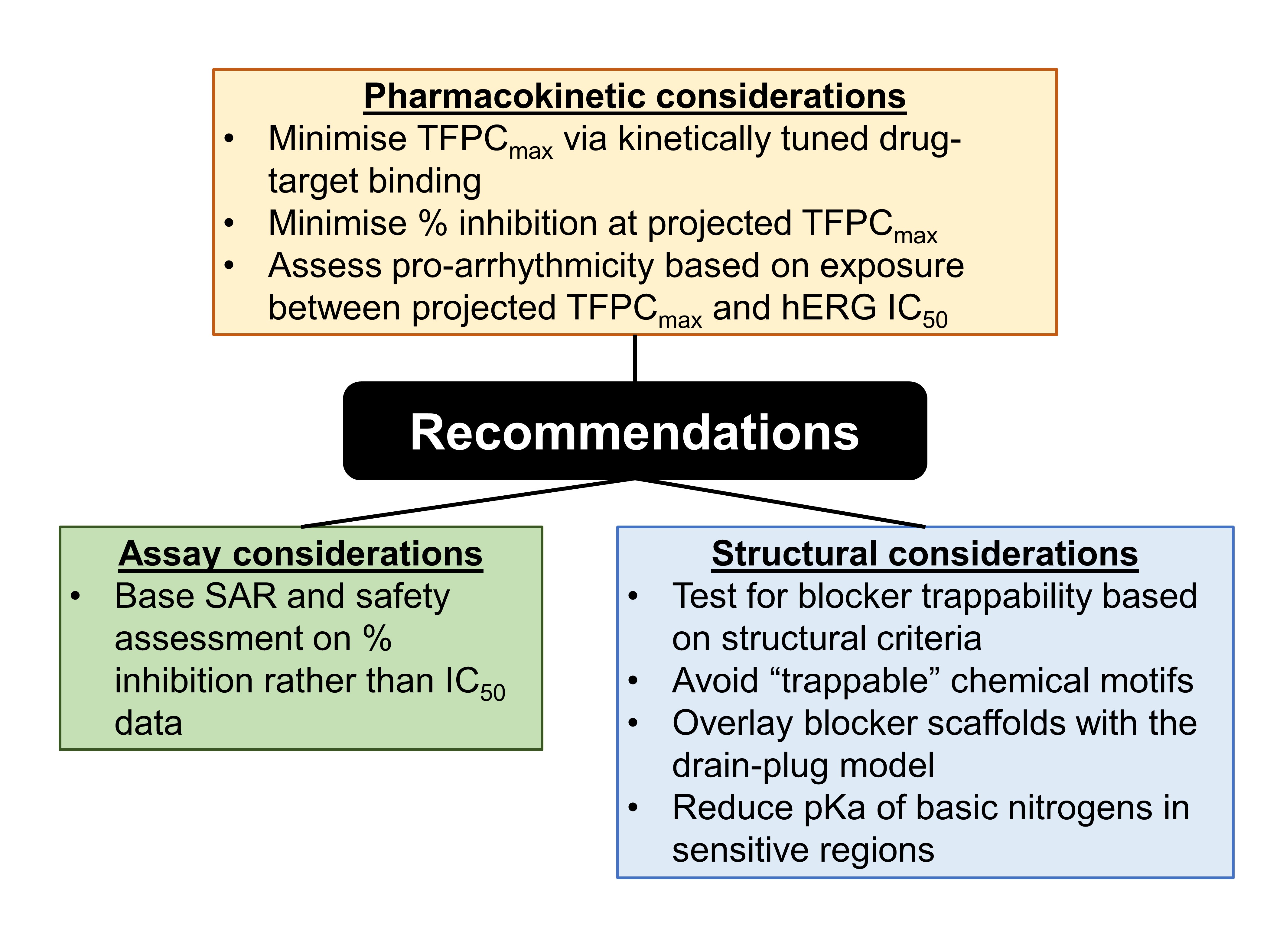
Figure 2. Recommendations by Wan et al. for testing.
What I like about these preprints
Despite the headway made by toxicologists and pharmaceutical scientists in developing in silico, in vitro, and in vivo models to predict or test for toxicity in drug candidates, the estimation of drug toxicity in humans remains a formidable challenge. Indeed, toxicity continues to be among the most common reasons for drug candidate failures [8]. Furthermore, the financial and clinical burden arising from ADRs clearly indicate the need for better toxicity predictions.
I selected these two preprints by Smit et al. and Wan et al. for their complementary approaches in mitigating this problem. Smit et al. used data analytics to sieve through two large global datasets (FAERS and SIDER). By considering activity across multiple targets, the authors improved the detection of AE-associated drugs in both datasets, thus demonstrating the orthogonality of information provided by different targets. In fact, this orthogonality extends to the SIDER and FAERS datasets as well—the authors found that the target-AE associations differ significantly between both databases. Trawling through such large amounts of data allowed the authors to generate multiple hypotheses on which further laboratory research could be centred.
Smit et al. also emphasised the importance of using plasma concentration data to enhance the predictive power of their analyses. Such a finding is not surprising: after all, plasma concentration data is literally the lifeblood of the pharmacokinetics field. For the work conducted by Smit et al. to gain greater regulatory and clinical acceptance, therefore, alternative methods to using in-human data to conduct such pharmacokinetic analyses will need to be established.
In the pharmaceutical industry, a few toxicities have regulatory and clinical implications: these include in vitro screens for carcinogenicity and developmental toxicity, as well as the cardiac, respiratory, and neurological organ systems. In their preprint, Wan et al. proposed an alternative method to improve the prediction of cardiac toxicity arising from hERG channel blockade: they identified target receptors, plasma concentrations, and structural properties commonly associated with hERG channel blockers, and offered some recommendations on how drug researchers might avoid these toxicities.
Future efforts will likely continue in the same vein; pharmacologists and toxicologists will continue to develop methods that allow them to draw better conclusions from precious, rare, in-human data. A common joke in pharmacology circles is that the word “pharmacy” cannot be spelled without the word “harm”. But with hard work and some luck, we might come to learn how better to reduce it.
References
[1] Sultana J, Cutroneo P, Trifirò G, Clinical and economic burden of adverse drug reactions, J Pharmacol Pharmacother 4(Suppl 1) (2013) S73-S77.
[2] Lazarou J, Pomeranz BH, Corey PN, Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies, Jama 279(15) (1998) 1200-1205.
[3] Krejsa CM, Horvath D, Rogalski SL, Penzotti JE, Mao B, Barbosa F, Migeon JC, Predicting ADME properties and side effects: the BioPrint approach, Curr Opin Drug Discov Devel 6(4) (2003) 470-480.
[4] Lynch JJ, 3rd, Van Vleet TR, Mittelstadt SW, Blomme EAG, Potential functional and pathological side effects related to off-target pharmacological activity, J Pharmacol Toxicol Methods 87 (2017) 108-126.
[5] Barber MJ, Wendt DJ, Starmer CF, Grant AO, Blockade of cardiac sodium channels. Competition between the permeant ion and antiarrhythmic drugs, The Journal of Clinical Investigation 90(2) (1992) 368-381.
[6] Whitebread S, Hamon J, Bojanic D, Urban L, Keynote review: in vitro safety pharmacology profiling: an essential tool for successful drug development, Drug Discov Today 10(21) (2005) 1421-1433.
[7] Bowes J, Brown AJ, Hamon J, Jarolimek W, Sridhar A, Waldron G, Whitebread S, Reducing safety-related drug attrition: the use of in vitro pharmacological profiling, Nat Rev Drug Discov 11(12) (2012) 909-922.
[8] Van Norman GA, Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach?, JACC: Basic to Translational Science 4(7) (2019) 845-854.
Sign up to customise the site to your preferences and to receive alerts
Register hereAlso in the pharmacology and toxicology category:
Snake venom metalloproteinases are predominantly responsible for the cytotoxic effects of certain African viper venoms
Daniel Osorno Valencia
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
In vitro pharmacokinetics and pharmacodynamics of the diarylquinoline TBAJ-587 and its metabolites against Mycobacterium tuberculosis
Zhang-He Goh
preLists in the pharmacology and toxicology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
COVID-19 / SARS-CoV-2 preprints
List of important preprints dealing with the ongoing coronavirus outbreak. See http://covidpreprints.com for additional resources and timeline, and https://connect.biorxiv.org/relate/content/181 for full list of bioRxiv and medRxiv preprints on this topic
| List by | Dey Lab, Zhang-He Goh |
1
Drug use in special populations
Any drugs that are being used in special populations: Patients with liver and kidney failure, in paediatrics, in geriatrics, and in pregnant or lactating patients. Includes the discovery of factors that could potentially affect drug use in these special populations.
| List by | Zhang-He Goh |
Toxicology of toxicants, existing therapeutics, and investigational drugs
Preprints that describe the toxicology of environmental pollutants and existing and upcoming drugs. Includes both toxicokinetics and toxicodynamics, as well as technological improvements that will help in the characterisation of this field.
| List by | Zhang-He Goh |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |
Anticancer agents: Discovery and clinical use
Preprints that describe the discovery of anticancer agents and their clinical use. Includes both small molecules and macromolecules like biologics.
| List by | Zhang-He Goh |
Advances in Drug Delivery
Advances in formulation technology or targeted delivery methods that describe or develop the distribution of small molecules or large macromolecules to specific parts of the body.
| List by | Zhang-He Goh |











 (No Ratings Yet)
(No Ratings Yet)