Toll-like receptor 4 is activated by platinum and contributes to cisplatin-induced ototoxicity
Posted on: 7 August 2020
Preprint posted on 20 June 2020
Article now published in EMBO reports at http://dx.doi.org/10.15252/embr.202051280
Application of the in vivo oxidative stress reporter Hmox1 as mechanistic biomarker of arsenic toxicity
Posted on:
Preprint posted on 20 June 2020
Article now published in Environmental Pollution at http://dx.doi.org/10.1016/j.envpol.2020.116053
Elements of elemental toxicity: researchers investigate the mechanisms underlying the toxicities of cisplatin and arsenic
Selected by Zhang-He GohCategories: pharmacology and toxicology
Background of preprints
Arsenic and platinum are elements that are highly toxic at low concentrations [1] that have risen to fame in the public eye for vastly different reasons. Arsenic, an element that commonly features in the plots of many murder stories, was a popular poison (and, somewhat ironically, medicine) throughout much of human history. Today, arsenic draws attention as an environmental pollutant from environmentalists, regulators, and citizens. In contrast, platinum is probably most known for its prevalence as a medicine today.
In line with the famed adage by Paracelsus that “the dose makes the poison”, both these elements have been—and are still being—used as medicines. Today, platinum is widely used in the anticancer drug cisplatin; arsenic is being used in its trioxide as an anticancer agent as well.
Given the wide-ranging medical and toxicological implications of these two elements, there is a need to probe the mechanisms underlying their toxicity. In their preprint, Babolmorad et al. homed in on the ototoxicity commonly associated with cisplatin (Fig. 1A), a platinum-containing anticancer agent. Specifically, the authors investigated the involvement of Toll-like Receptor 4 (TLR4), a membrane-bound pattern recognition receptor, in the signalling pathway of cisplatin toxicity.
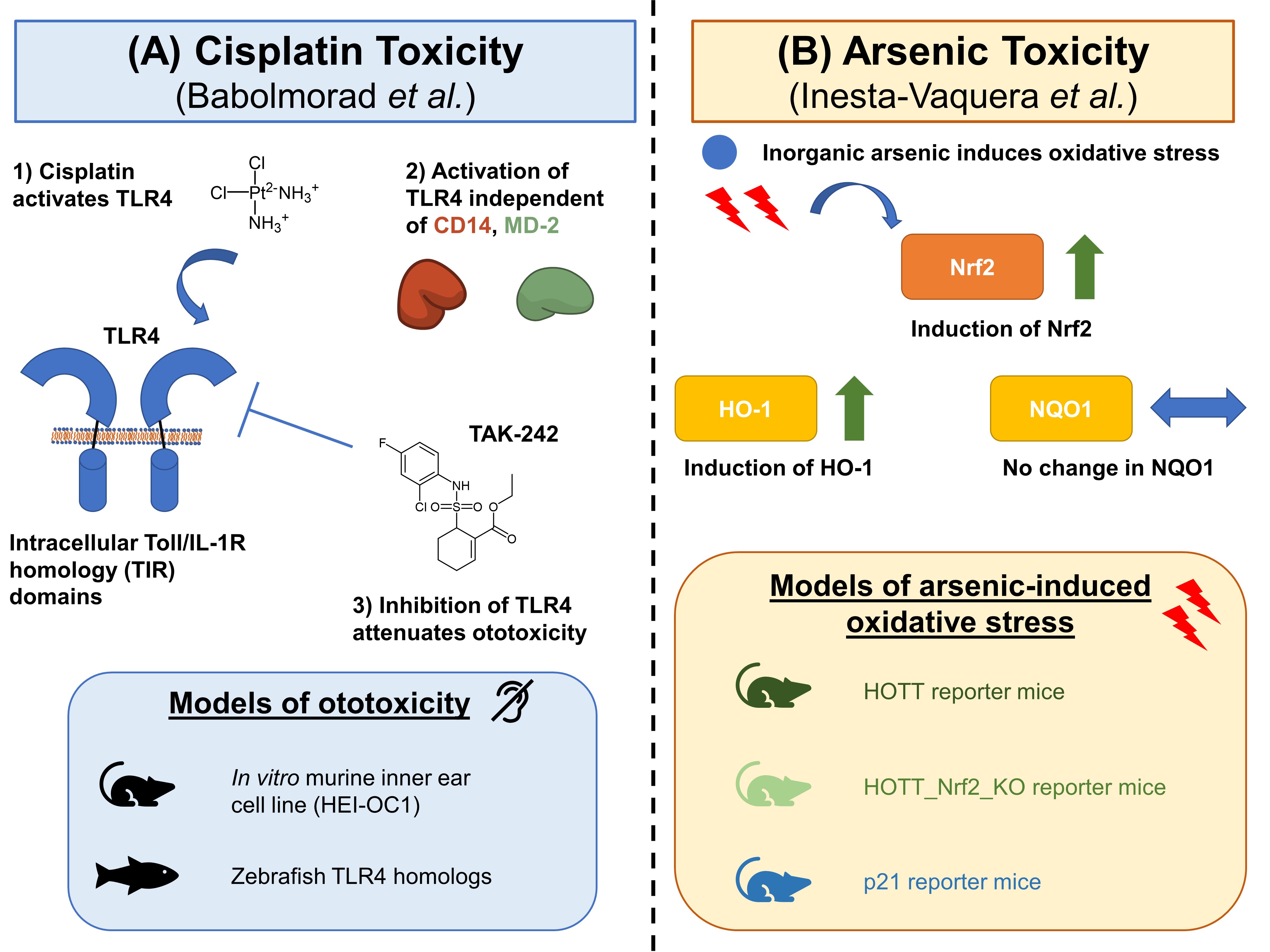
Figure 1. Graphical summary of the preprints discussed in this preLight.
In the preprint by Inesta-Vaquera et al., the authors probed the role of Nrf2 in inorganic arsenic toxicity (Fig. 1B). Nrf2 is a transcription factor that, together with Kelch-like ECH-associated protein 1 (KEAP1), is involved in a major antioxidant pathway. The authors probed the role of this pathway in inorganic arsenic toxicity using two mouse models: a Hmox1 reporter of oxidative stress/inflammation (HOTT), as well as a p21 reporter that measures DNA damage.
Key findings of preprints
(A) Babolmorad et al.
Babolmorad et al. used HEK reporter cell lines to show that, like the toxicity induced by other transition metals [2], both platinum and cisplatin induce toxicity by activating TLR4. Indeed, a rescue experiment involving the inhibition of TLR4 using the inhibitor TAK-242 further reinforced these findings.
Interestingly, the activation of TLR4 by cisplatin did not proceed through the same canonical pathway through which TLR4 is activated by gram-negative bacterial surface component lipopolysaccharide (LPS), its usual binding partner. LPS usually activates TLR4 by also binding CD14 and MD-2 co-receptors. In contrast, the authors observed that cisplatin’s activation of TLR4 does not involve CD14 and MD-2 co-receptors.
Babolmorad et al. then further strengthened their findings using two further experiments—one in vitro and the other in vivo. The authors first showed that deleting TLR4 in a mouse inner ear Organ of Conti cell line HEI-OC1 reduced cisplatin-induced ototoxicity. They then performed knockdowns of TLR4 homologues in zebrafish and found that these knockdowns also protected against cisplatin-induced ototoxicity.
(B) Inesta-Vaquera et al.
Inesta-Vaquera et al. orally dosed the HOTT mice with inorganic arsenic at two doses: a low dose to simulate chronic environmental exposure in real life; and an acute high dose to allow the authors to make comparisons to current literature reports. This experiment corroborated current evidence [3] that the liver, kidney, and heart indeed exhibit a high reporter response when exposed to inorganic arsenic. Interestingly, when the authors exposed the p21 reporter mice to an acute high dose of inorganic arsenic, they observed no further activation of the reporter, leading them to conclude that the initial oxidative stress from inorganic arsenic does not trigger a DNA damage response.
Next, Inesta-Vaquera et al. characterised the epigenetic changes in their mouse line by chronically exposing HOTT mice to inorganic arsenic. This led to a significant hypomethylation in the total DNA methylation of selected genes (SOCS3, EGR1, JUNB, and DUSP1) in a tissue-specific manner. To show that the induction of Hmox1 occurs via Nrf2 activation by inorganic arsenic, the authors also generated a line of HOTT reporter_Nrf2-knockout mice. They found that inorganic arsenic did not activate the HOTT reporter in the HOTT reporter_Nrf2-knockout mice, suggesting that Nrf2 plays a key role in the induction of HOTT by inorganic arsenic.
Finally, the authors conducted a rescue experiment by co-administering the inorganic arsenic with antioxidant N-acetylcysteine (NAC). NAC protected tissues against oxidative stress induced by inorganic arsenic, leading the authors to conclude that their HOTT reporter mouse model could help drive further research in reducing arsenic toxicity.
What I like about these preprints: implications and future work
A common theme that unites both preprints that I have discussed in this preLight is the physiological and pathophysiological role of oxidative stress. The preprint by Babolmorad et al. focussed on the role of TLR4 in the modulation of the inflammatory response—which is upstream of oxidative stress. Indeed, Babolmorad et al. pointed out that ROS generation and apoptosis induction have been implicated in cisplatin’s ototoxicity.
In the preprint by Inesta-Vaquera et al., oxidative stress is implicated even more directly. The authors studied the oxidative stress mechanisms underlying the toxicity of inorganic arsenic, and identified Nrf2 and Hmox1 as key mediators in these mechanisms.
Both preprints have important clinical implications. By understanding the mechanisms of toxicity, researchers will be better able to design protectants as antidotes: these may come in the form of otoprotectants in the case of TLR4-mediated ototoxicity, and antioxidants to mitigate inorganic arsenic-induced toxicity.
Understanding these mechanisms of toxicity will also enable future drug development. By using various models—for example, the zebrafish model—to test for ototoxicity in their preprint, Babolmorad et al. expanded the literature and inform future efforts to use these same models to test preclinical candidates for ototoxicity. Similarly, the HOTT mouse model described by Inesta-Vaquera et al. could also help pharmacologists and toxicologists rapidly identify drugs that may be associated with oxidative stress. Researchers working in drug development may use these models to more quickly construct structure-toxicity relationships, accelerating the drug discovery process and making it a safer endeavour.
In addition to strengthening pharmaceutical regulation, the findings discussed in these preprints are also pertinent to public health measures. Babolmorad et al. noted similarities between the toxicity of platinum and immune hypersensitivity reactions to the transition metals nickel, cobalt, and palladium; nickel and cobalt are known environmental pollutants, so identifying the consequences of transition metal-poisoning may help in the implementation of regulations to contain the spread of these pollutants and treat hapless patients who have been exposed. Arsenic exposure, which is also a common health threat to millions of people worldwide, may similarly benefit.
These scientific gains made in the understanding of element toxicity are the latest to join decades of medical and environmental research. I am hopeful that they will inform and support policies in healthcare and environmentalism, and guide us towards a cleaner, greener, and safer world.
Open questions
(A) Babolmorad et al.
- It is interesting that cisplatin can activate TLR4 via a non-canonical pathway, i.e. it does not require the co-receptors MD-2 and CD14 for binding. What might be a structural or biophysical reason for this? What implications might it have for the development of antidotes that would help to mitigate this toxicity?
(B) Inesta-Vaquera et al.
- In addition to Hmox1, other antioxidant response elements have also been implicated in the Nrf2 pathway. Some examples of these are [4]: Glutamate-cysteine ligase catalytic subunit (Gclc), NAD(P)H quinone dehydrogenase 1 (NQO1), Sulfiredoxin 1 (Srxn1), and Thioredoxin 1 (Trx1). Other than NQO1, did you observe the modulation of any of these other proteins when you tested for the effects of inorganic arsenic?
- Other than with TBE-31, Nrf2 induction has also been implicated in the cytoprotective mechanisms of certain agents. For example, sulforaphane is a known inducer of Nrf2 [5]. Was the HOTT model also tested with other Nrf2 inducers that do not induce oxidative stress?
References
[1] Hunter P, Essentially deadly: living with toxic elements: Humans and plants have evolved various mechanisms to deal with and even adopt toxic heavy metals, EMBO Rep 16(12) (2015) 1605-1608.
[2] Rachmawati D, Bontkes HJ, Verstege MI, Muris J, von Blomberg BM, Scheper RJ, van Hoogstraten IM, Transition metal sensing by Toll-like receptor-4: next to nickel, cobalt and palladium are potent human dendritic cell stimulators, Contact Dermatitis 68(6) (2013) 331-338.
[3] Thomas DJ, Styblo M, Lin S, The cellular metabolism and systemic toxicity of arsenic, Toxicol Appl Pharmacol 176(2) (2001) 127-144.
[4] Hawkins Kate E, Joy S, Delhove Juliette MKM, Kotiadis Vassilios N, Fernandez E, Fitzpatrick Lorna M, Whiteford James R, King Peter J, Bolanos Juan P, Duchen Michael R, Waddington Simon N, McKay Tristan R, NRF2 Orchestrates the Metabolic Shift during Induced Pluripotent Stem Cell Reprogramming, Cell Reports 14(8) (2016) 1883-1891.
[5] Zhou R, Lin J, Wu D, Sulforaphane induces Nrf2 and protects against CYP2E1-dependent binge alcohol-induced liver steatosis, Biochimica et Biophysica Acta (BBA) – General Subjects 1840(1) (2014) 209-218.
Acknowledgements
The preLight author is grateful to Babolmorad et al. for their suggestions regarding Figure 1.
Sign up to customise the site to your preferences and to receive alerts
Register hereAlso in the pharmacology and toxicology category:
Snake venom metalloproteinases are predominantly responsible for the cytotoxic effects of certain African viper venoms
Daniel Osorno Valencia
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
In vitro pharmacokinetics and pharmacodynamics of the diarylquinoline TBAJ-587 and its metabolites against Mycobacterium tuberculosis
Zhang-He Goh
preLists in the pharmacology and toxicology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
COVID-19 / SARS-CoV-2 preprints
List of important preprints dealing with the ongoing coronavirus outbreak. See http://covidpreprints.com for additional resources and timeline, and https://connect.biorxiv.org/relate/content/181 for full list of bioRxiv and medRxiv preprints on this topic
| List by | Dey Lab, Zhang-He Goh |
1
Drug use in special populations
Any drugs that are being used in special populations: Patients with liver and kidney failure, in paediatrics, in geriatrics, and in pregnant or lactating patients. Includes the discovery of factors that could potentially affect drug use in these special populations.
| List by | Zhang-He Goh |
Toxicology of toxicants, existing therapeutics, and investigational drugs
Preprints that describe the toxicology of environmental pollutants and existing and upcoming drugs. Includes both toxicokinetics and toxicodynamics, as well as technological improvements that will help in the characterisation of this field.
| List by | Zhang-He Goh |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |
Anticancer agents: Discovery and clinical use
Preprints that describe the discovery of anticancer agents and their clinical use. Includes both small molecules and macromolecules like biologics.
| List by | Zhang-He Goh |
Advances in Drug Delivery
Advances in formulation technology or targeted delivery methods that describe or develop the distribution of small molecules or large macromolecules to specific parts of the body.
| List by | Zhang-He Goh |











 (No Ratings Yet)
(No Ratings Yet)