Cerebellar contribution to preparatory activity in motor neocortex
Posted on: 17 July 2018 , updated on: 18 July 2018
Preprint posted on 31 May 2018
Article now published in Neuron at http://dx.doi.org/10.1016/j.neuron.2019.05.022
Chabrol and colleagues demonstrate that cerebellar activity rapidly modulates movement related preparatory activity in the motor neocortex, half-way across the brain.
Selected by Mahesh KarnaniCategories: neuroscience
Context
We do not yet know how the mammalian brain orchestrates movement and how brain-wide changes in neuronal activity occur during and before movements. It is well established that the cerebellum is important for learning and executing fine motor tasks, and that there is preparatory activity in the motor neocortex before movements are initiated. These interact via the motor thalamus and the basal pontine nucleus, and previous work has demonstrated control of motor neocortex by optogenetic activation of cerebellar Purkinje cells1. However, it is unclear how the cerebellum participates in neocortical activity outside the context of fine motor control, and it has not been shown how the cerebellum modulates sensory task related activity in the motor neocortex.
To address these issues Chabrol and colleagues set up a sensorimotor virtual reality task for head-fixed mice so that neuronal activity could be recorded with silicon probes during goal-directed movement. Neuronal firing was recorded from three sites along the cerebellar-motor neocortex pathway while trained mice performed the task. The task required mice to run along a virtual corridor and stop after a particular visual cue displayed on the virtual walls in order to get a soy milk reward through a licking spout. This particular task is a brilliant choice because it incorporates two types of movement, locomotion and licking. In principle, the results can therefore contribute to two related topics: preparatory activity2 which is commonly studied in subjects that withhold motion until a start cue, and brain control of locomotion3,4.
Key findings
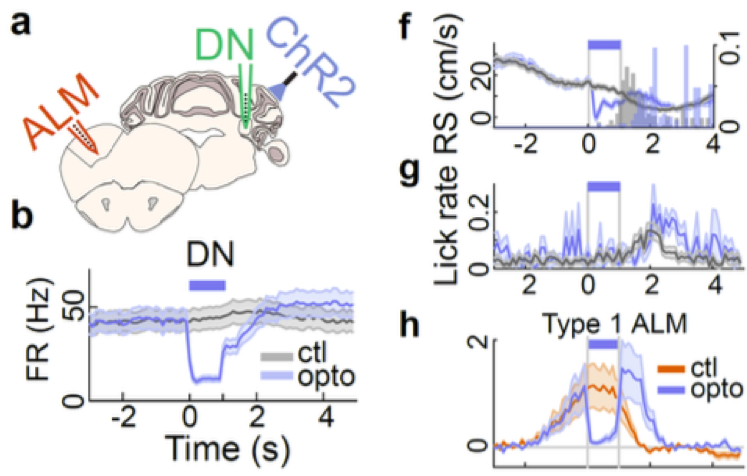
The main findings in this preprint arise from activity histograms aligned to the time point where the mouse stops in the reward zone and increases licking as it receives the reward. In more detail, running speed gradually decreases and licking rate increases in the 2 seconds before this time point. Unit recordings from the dentate nucleus (DN, a cerebellar output nucleus), lateral crus 1 cerebellar cortex and anterolateral motor neocortex (ALM) revealed neurons that have distinct activity profiles around reward delivery. Approximately half of the neurons in DN and ALM fell into three main categories of preparatory activity2: ones that ramp up activity during the 2 seconds before reward (type 1), ones that ramp up and then increase further after reward delivery (type 2) and ones that only increase activity after reward delivery (type 3). In contrast, output neurons of the cerebellar cortex decreased firing on average during the 2 seconds before reward.
The firing patterns suggested to the authors that the inhibitory cerebellar cortex neurons, by decreasing their activity, may allow DN to excite ALM, which is located more than half-way across the brain. The authors put this to the test from the reverse perspective by optogenetically activating the cerebellar cortex Purkinje neurons, and found that this rapidly decreased firing of the majority of DN and ALM neurons showing preparatory activity. While this manipulation did not have an immediate effect on licking rate, the mice decreased running speed during the optogenetic light pulse, causing them to reach the reward later in the trial. This suggests that these neurons, that seemed to be involved in organizing licking (due to their activity patterns most closely mirroring lick rate), might be crucial for coordinating goal-directed locomotion and licking. The recordings in the cerebellar cortex were targeted to a part of crus 1 that responds to activation of primary visual cortex and limb motor cortex, to hone in on the faculties relevant to the task. However, it is unclear whether the findings could be generalizable to the rest of the cerebellum as well.
Why I chose this preprint
Part of my current project involves neural control of locomotion, so I was delighted when my daily bioRxiv subject collection email alert pointed out such a competent piece of related science, fresh off the bench and shared openly via bioRxiv. I was initially attracted to the optogenetic manipulation experiment showing that cerebellar crus 1 Purkinje cells can rapidly control ongoing goal-directed locomotion. This is a small part of the study, though, and the simple conclusion that this circuit controls locomotion is confounded by the plethora of likely downstream effects on cortical, thalamic and rubrospinal circuits.
The main message of the study is that the cerebellum is part of the brain wide circuit that produces preparatory activity. The results show clearly that cerebellar disinhibition modulates motor planning related neuronal activity in a neocortical area more than half-way across the brain. As a firm believer in the importance local microcircuits, such a potent long-range effect gives me a welcome chance to reflect on my beliefs.
What next?
This and other studies2 have shown that preparatory activity arises in widely distributed, interdependent motor brain regions. The next challenge should be to find how these circuits interact to turn preparatory activity into executable movements. Numerous recent studies have demonstrated profound contributions to normal locomotion from key neuronal populations. Optogenetics has allowed the necessary millisecond precision control of these neurons to assess their contributions. Recently, contributions to locomotion from midbrain dopamine neurons5, mesencephalic locomotor region neurons6, caudal brainstem neurons7 and cerebellar lobule V Purkinje cells8 have been dissected primarily with optogenetics. These add to a growing description of brain control of locomotion3,4. Comparing some of these studies to this preprint raises some general questions: If an optogenetic manipulation stops an animal from moving, is that enough to conclude that the affected cells control locomotion, or is more detailed knowledge of the activity of other locomotion controlling neurons required? Is goal-directed locomotion controlled by different circuits than non-goal-directed locomotion, i.e., self-paced running? Would the activity profiles of the neurons recorded in this preprint, for example, be substantially different during non-goal-oriented movements? And would their manipulation outside the task yield similar results? Perhaps these questions would necessitate another study with similarly rigorous recordings. Still, it is wonderful that the authors chose an experimental paradigm that can contribute to two fields at once.
References:
- Proville, R. D. et al. Cerebellum involvement in cortical sensorimotor circuits for the control of voluntary movements. Nat. Neurosci. 17, 1233–9 (2014).
- Svoboda, K. & Li, N. Neural mechanisms of movement planning: motor cortex and beyond. Curr. Opin. Neurobiol. 49, 33–41 (2018).
- Kim, L. H. et al. Integration of Descending Command Systems for the Generation of Context-Specific Locomotor Behaviors. Front. Neurosci. 11, 581 (2017).
- Gatto, G. & Goulding, M. Locomotion Control: Brainstem Circuits Satisfy the Need for Speed. Curr. Biol. 28, R256–R259 (2018).
- da Silva, J. A., Tecuapetla, F., Paixão, V. & Costa, R. M. Dopamine neuron activity before action initiation gates and invigorates future movements. Nature 554, 244–248 (2018).
- Caggiano, V. et al. Midbrain circuits that set locomotor speed and gait selection. Nature 553, 455–460 (2018).
- Capelli, P., Pivetta, C., Soledad Esposito, M. & Arber, S. Locomotor speed control circuits in the caudal brainstem. Nature 551, 373–377 (2017).
- Hoogland, T. M., De Gruijl, J. R., Witter, L., Canto, C. B. & De Zeeuw, C. I. Role of Synchronous Activation of Cerebellar Purkinje Cell Ensembles in Multi-joint Movement Control. Curr. Biol. 25, 1157–65 (2015).
Sign up to customise the site to your preferences and to receive alerts
Register hereAlso in the neuroscience category:
PPARδ activation in microglia drives a transcriptional response that primes phagocytic function while countering inflammatory activation
Isabel Paine
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Self-renewal of neuronal mitochondria through asymmetric division
Lorena Olifiers
preLists in the neuroscience category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |











 (No Ratings Yet)
(No Ratings Yet)