Accelerated erythrocyte senescence causes dose-limiting anemia of antimalarial enolase inhibitors
Posted on: 31 October 2020 , updated on: 1 November 2020
Preprint posted on 10 October 2020
Hallow-ENO: selectively targeting erythrocyte glycolysis improves antimalarial efficacy and accelerates erythrocyte senescence.
Selected by Zhang-He GohCategories: pharmacology and toxicology
Background of preprint
Malaria, an infectious disease caused by Plasmodium parasites, takes a massive toll on morbidity and mortality. While a positive selective pressure on hosts with certain hereditary blood disorders has been observed in certain malaria-endemic regions, efforts are still ongoing to understand the underlying mechanisms.
To circumvent problems associated with current inhibitors, such as the lack of specificity in target inhibition, Jezewski et al. target enolase to achieve cellular specificity of glycolytic inhibition. Enolase, an enzyme that catalyses the eighth step in glycolysis, exists as three isoforms in humans—ENO1(in all cell types), ENO2 (in neurons and erythrocytes), and ENO3 (in muscles)—which the authors utilised (Fig. 1). First, the authors characterised the physiologic effects of glycolytic inhibition in erythrocytes, and then they measured the impact of acute and specific glycolytic inhibition of erythrocytes on Plasmodium infection.
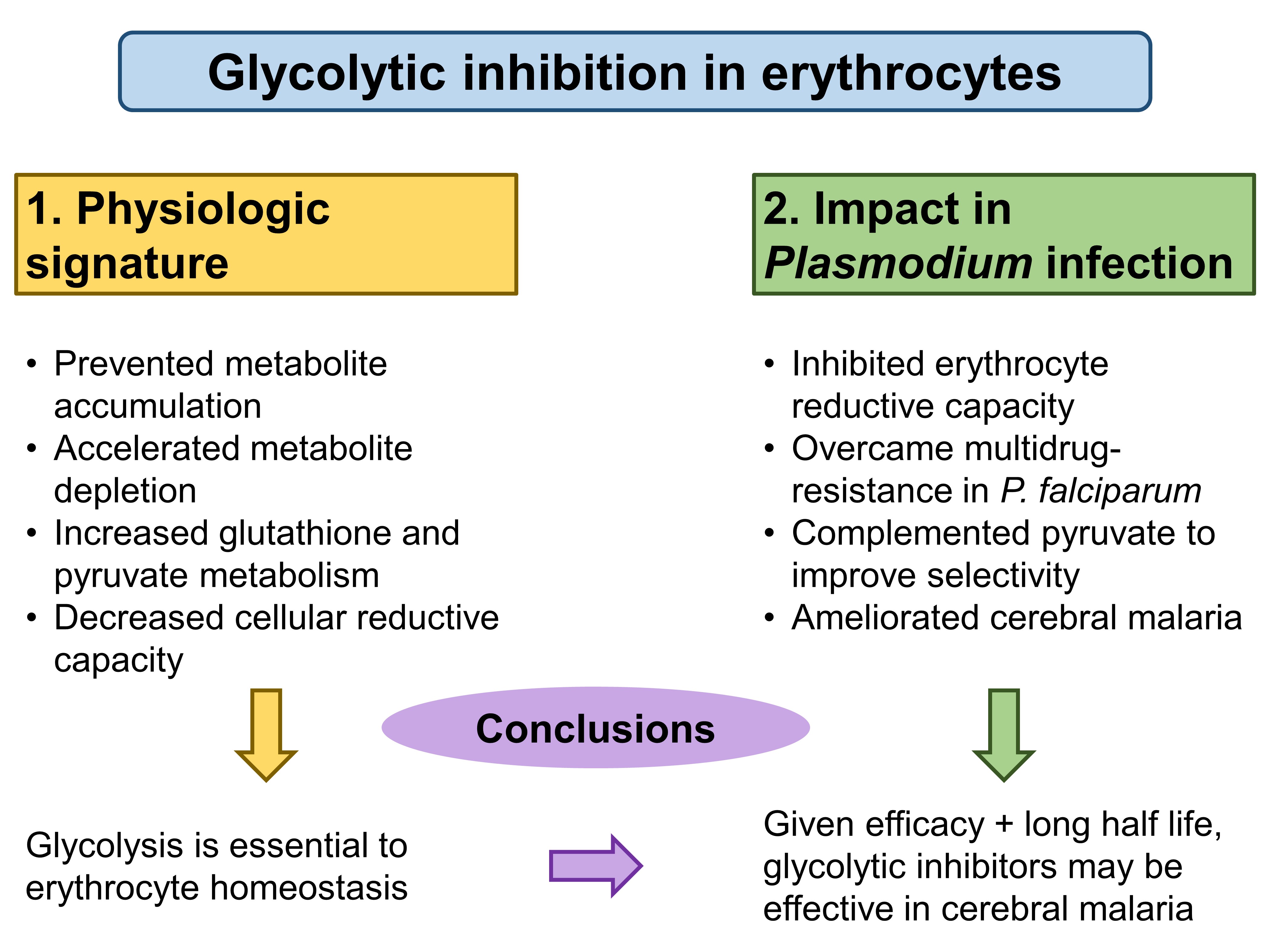
Figure 1. A summary of glycolytic inhibition effects in the preprint by Jezewski et al.
Key findings of preprint
(A) Metabolic profile of disrupted glycolysis in erythrocytes
First, Jezewski et al. used metabolomics to validate two lipophilic ester prodrugs, POM-SF and POM-HEX, establishing that treatment of erythrocytes with these two agents led to distinct metabolic profiles from untreated controls. These studies implicated enolase as the key mediator in the glycolytic inhibition. The generated profiles arising from glycolytic inhibition largely fell into two clusters: the prevention of metabolite accumulation, and the acceleration of metabolite depletion.
Specifically, the authors found that acute glycolytic inhibition increased glutathione and pyruvate metabolism. Since glutathione and pyruvate are antioxidants, glycolytic inhibition limited cells’ reductive capacity and increased their susceptibility to oxidative stress. Indeed, the authors confirmed a dose-dependent loss of reductive capacity using 13C-labelled experiments, and further showed that the increased susceptibility to oxidative stress led to accelerated senescence in erythrocytes.
From these experiments, Jezewski et al. concluded that glycolysis is essential to erythrocyte homeostasis.
(B) Impact of antiglycolytic inhibition in Plasmodium infection
Because Plasmodium induces oxidative stress in infected erythrocytes [1-3], the authors hypothesised that inhibiting erythrocyte reductive capacity could result in protection against malaria. To verify this hypothesis, the authors directly tested the antiparasitic activity of erythrocyte glycolysis inhibitors. Significantly, the antimalarial potency of these compounds correlated well with their ability to disrupt erythrocyte redox balance in vitro (and slightly less strongly in vivo in mice), leading the authors to conclude that erythrocyte enolase is likely the common target that links these two characteristics.
Next, Jezewski et al. verified that POM-SF and POM-HEX are effective against multidrug-resistant P. falciparum, and that further selectivity could be achieved with chemical complementation with pyruvate. The authors also found that enolase inhibition using selective inhibitors could reduce parasite burden and improve clinical scores and survival in murine cerebral malaria models.
Finally, the authors also investigated the pharmacodynamic and pharmacokinetic properties of enolase inhibitor HEX in non-human primates. Their experiments demonstrated that serum concentrations remained above the in vitro EC50 against Plasmodium for at least 4 hours, leading them to conclude that these agents are likely to be clinically efficacious.
Why we highlighted this preprint
At the end of 2018, one of us (Zhang-He) wrote a preLight about the contributions of various drug discovery advances in the field of malaria. Indeed, the control of malaria has come a long way since then (though the ongoing covid-19 pandemic has unfortunately set progress back significantly [4]).
We selected this preprint to highlight the presentation of haemotoxicity in antimicrobial treatments. Many antimalarial treatments—such as primaquine and chloroquine—are often accompanied by haemotoxicities. In this preprint, the evidence presented by Jezewski et al. appears to suggest that such a trade-off indeed exists for antimalarials, particularly with therapy targeting host factors.
Despite these considerations, we believe that host factors in erythrocytes are promising drug targets for the reasons stated in the preprint: (a) erythrocytes serve as a vital host to the malaria life cycle and (b) variation in erythrocyte function in certain diseases confer a selective advantage to the host, suggesting multiple potential drug targets for malaria treatment.
Additionally, some selectivity may be possible. While oxidative stress is the very mechanism of action underlying both antimalarials’ efficacy and toxicity, the authors wrote that there may be some room for selectivity.
In this preprint, the authors posited that the human enolase is the main target of the inhibitors’ mechanism of action; but they also noted that they could not rule out other targets. That is probably what future work will build on—validating this target and building inhibitors to selectively target it over other isoforms. Further optimisation of the inhibitors’ pharmacokinetics will also be performed to improve their clinical utility.
The spooky season may be upon us, but the resistance to existing antimalarials remains a real and persistent threat. Against this ominous backdrop, we fervently hope that the discovery of these new druggable targets will give mankind a fillip in our constant battle against malaria.
Open questions
- Were any markers of oxidative stress in vivo measured, and how well did these correlated with the inhibitor-induced anaemia in vivo?
- Were there differences in the metabolic profiles of erythrocytes treated with POM-SF versus POM-HEX? For example, was one more efficacious in inhibiting glycolysis? Did this increase in efficacy affect the erythrocyte senescence or resistance to malaria infection?
- What is the clinical impact of accelerated erythrocyte senescence on anaemia?
- Given that neurons and erythrocytes both rely on enolase isoform ENO2 for glycolysis, was there a reason why murine cerebral malaria was chosen as the in vivo model?
- Given that it is dose-dependent, is the negative impact of enolase inhibition transient? Specifically, do erythrocytes return to their normal rates of reaching senescence when the drug is washed out? This may be clinically significant, because patients who develop anaemia on the drug can be managed temporarily with blood transfusions for the course of the treatment.
- From a clinical perspective, do you foresee any neurological side effects from inhibiting ENO2?
References
[1] Cyrklaff M, Srismith S, Nyboer B, Burda K, Hoffmann A, Lasitschka F, Adjalley S, Bisseye C, Simpore J, Mueller A-K, Sanchez CP, Frischknecht F, Lanzer M, Oxidative insult can induce malaria-protective trait of sickle and fetal erythrocytes, Nature Communications 7(1) (2016) 13401.
[2] Mohan K, Ganguly NK, Dubey ML, Mahajan RC, Oxidative damage of erythrocytes infected with Plasmodium falciparum, Annals of Hematology 65(3) (1992) 131-134.
[3] Becker K, Tilley L, Vennerstrom JL, Roberts D, Rogerson S, Ginsburg H, Oxidative stress in malaria parasite-infected erythrocytes: host-parasite interactions, Int J Parasitol 34(2) (2004) 163-189.
[4] Sherrard-Smith E, Hogan AB, Hamlet A, Watson OJ, Whittaker C, Winskill P, Ali F, Mohammad AB, Uhomoibhi P, Maikore I, Ogbulafor N, Nikau J, Kont MD, Challenger JD, Verity R, Lambert B, Cairns M, Rao B, Baguelin M, Whittles LK, Lees JA, Bhatia S, Knock ES, Okell L, Slater HC, Ghani AC, Walker PGT, Okoko OO, Churcher TS, The potential public health consequences of COVID-19 on malaria in Africa, Nature Medicine 26(9) (2020) 1411-1416.
Acknowledgements
This preLight was co-written with Teng Hiang Heng, a PhD student at the Wellcome Sanger Institute and the University of Cambridge. She graduated from the National University of Singapore, Yong Loo Lin School of Medicine and previously did her clinical rotations in the National University Health System.
Teng Hiang is also affiliated with the Agency for Science, Technology and Research (A*STAR) Singapore, where she did research attachments at the Singapore Immunology Network (SIgN). Her broad research interests include genomics, haematology, immunology, infectious diseases, cancer and paediatrics.
doi: https://doi.org/10.1242/prelights.25537
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the pharmacology and toxicology category:
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
In vitro pharmacokinetics and pharmacodynamics of the diarylquinoline TBAJ-587 and its metabolites against Mycobacterium tuberculosis
Zhang-He Goh
Beyond venomous fangs: Uloboridae spiders have lost their venom but not their toxicity
Daniel Fernando Reyes Enríquez, Marcus Oliveira
preLists in the pharmacology and toxicology category:
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
COVID-19 / SARS-CoV-2 preprints
List of important preprints dealing with the ongoing coronavirus outbreak. See http://covidpreprints.com for additional resources and timeline, and https://connect.biorxiv.org/relate/content/181 for full list of bioRxiv and medRxiv preprints on this topic
| List by | Dey Lab, Zhang-He Goh |
1
Drug use in special populations
Any drugs that are being used in special populations: Patients with liver and kidney failure, in paediatrics, in geriatrics, and in pregnant or lactating patients. Includes the discovery of factors that could potentially affect drug use in these special populations.
| List by | Zhang-He Goh |
Toxicology of toxicants, existing therapeutics, and investigational drugs
Preprints that describe the toxicology of environmental pollutants and existing and upcoming drugs. Includes both toxicokinetics and toxicodynamics, as well as technological improvements that will help in the characterisation of this field.
| List by | Zhang-He Goh |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |
Anticancer agents: Discovery and clinical use
Preprints that describe the discovery of anticancer agents and their clinical use. Includes both small molecules and macromolecules like biologics.
| List by | Zhang-He Goh |
Advances in Drug Delivery
Advances in formulation technology or targeted delivery methods that describe or develop the distribution of small molecules or large macromolecules to specific parts of the body.
| List by | Zhang-He Goh |











 (1 votes)
(1 votes)