Development and validation of serological markers for detecting recent exposure to Plasmodium vivax infection
Posted on: 31 December 2018 , updated on: 29 September 2019
Preprint posted on 30 November 2018
Antimalarial drug mefloquine kills both trophozoite and cyst stages of Entamoeba
Posted on: , updated on: 29 September 2019
Preprint posted on 20 December 2018
It’s in the blood: enhancing malarial detection and expanding the antimalarial toolbox to treating amoebiasis
Selected by Zhang-He GohCategories: microbiology, pharmacology and toxicology
Background of preprints
Over a week ago, Dr Pedro Alonso, the Director of the WHO Global Malaria Programme, released a newsletter that discussed selected areas of WHO’s World Malaria Report published one month earlier. Dr Alonso pointed out that while some countries with a high malarial burden made significant progress, the worldwide fight against malaria appears to have slowed. One such obstacle is the diagnostic gap in national malaria control programs, on which Longley et al. focussed. Specifically, Longley et al. believe that “identifying and targeting individuals with hypnozoites is… essential for accelerating malaria elimination”, and that “blanket approaches to malaria control and elimination” may not be efficient in coping with decreased endemicity as we approach the endgame of our battle with malaria. Longley et al. chose to work on Plasmodium vivax owing to its intractability.
Sauvey et al. examined the activity of mefloquine, an existing antimalarial, against Entamoeba histolytica, a parasitic amoeba which causes disease that is rampant in populations with sanitation problems. Because mefloquine is active against blood-stage malarial parasites and is also able to cross the blood-brain barrier, Sauvey et al. hypothesised that mefloquine may be useful for persistent invasive infections with a reservoir of parasites in the lumen; that is, mefloquine would be useful for treating E. histolytica infections.
Key findings of preprints
(A) Longley et al.
The findings in the preprint by Longley et al. can be broadly classified into three phases. First, Longley et al. narrowed down to 60 candidate serological markers in the antigen discovery phase, then validated them in the next phase. Longley et al. provided a summary of these two phases in Figure 2 of their preprint.
The authors first limited the infections to PCR-confirmed infections to those which were recent, defined as those within the last 9 months. They then attempted to classify individuals based on levels of various serological exposure markers (SEM). Using this method, the authors found that the reticulocyte binding protein 2b (RBP2b), labelled in their preprint as PVX_094255, was 74% sensitive and 74% specific when used alone. The authors then used a linear discriminant analysis (LDA) classification algorithm to identify combinations of the 60 candidate SEMs to predict infections, finding a degree of redundancy among these combinations, as summarised in Table 1 below.
Table 1. Top eight most frequently identified SEMs.
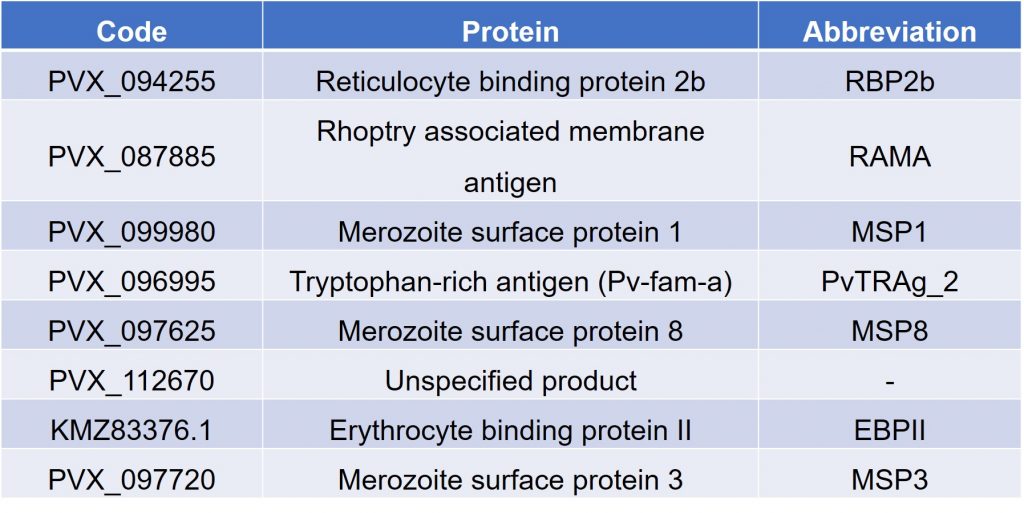
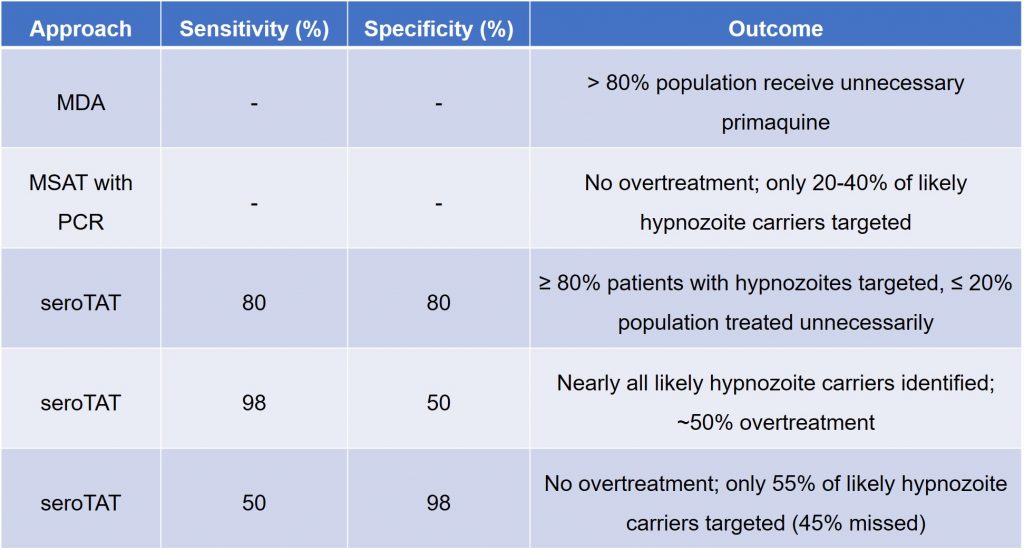
Finally, Longley et al. assessed the classification performance of their LDA classification algorithm. They found that as the number of proteins increased, classification performance plateaued at 80% sensitivity and 80% specificity with five proteins being used in classification. The authors then modelled the performance of targeted treatment conducted with the eight SEMs shown in Table 1, comparing the use of the seroTAT approach to both mass drug administration (MDA) and mass screening and treatment (MSAT) with PCR approaches. The findings can be summarised in Table 2 below.
Table 2. Performance of the seroTAT approach compared to current approaches.
(B) Sauvey et al.
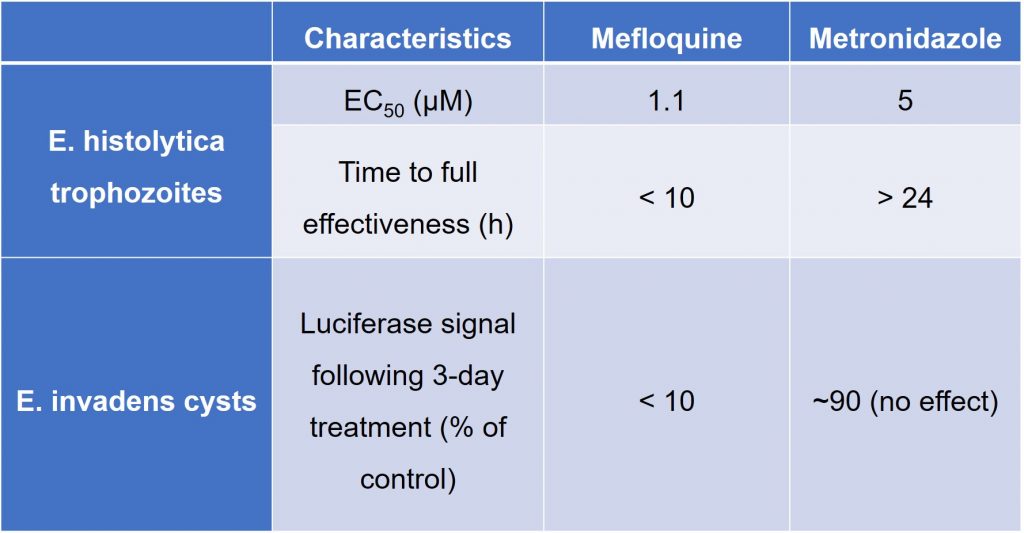
Using a cell viability assay to determine drug potency against E. histolytica, Sauvey et al. found that mefloquine is active against E. histolytica trophozoites in vivo. In the same assay system, the EC50 of mefloquine was determined to be 1.1 μM. Further investigation using a trypan blue cell exclusion assay showed that this decrease in cell viability could be attributed to mefloquine’s amoebicidal nature against E. histolytica. Sauvey et al. also observed that E. histolytica morphology changed significantly after mefloquine treatment, describing the appearance as “universally swollen and rounded, with dramatically enlarged vacuoles”. Subsequent time-effect experiments showed that mefloquine also killed E. histolytica trophozoites much more quickly than did metronidazole. Table 3 below summarises key characteristics for comparison between mefloquine and metronidazole.
Table 3. Characteristics of mefloquine compared to metronidazole.
Why I chose these preprints
I selected both these preprints in this preLight to wrap up 2018 as they encapsulate the recurring themes that I have written about this year: on modelling and prediction, on infectious diseases, on pharmacokinetics and toxicokinetics, and on drug discovery and repurposing. Furthermore, in highlighting these preprints (which have the topic of malaria in common), I aim to emphasise that while this blood-borne disease may be controlled, it is far from being eradicated.
I chose the preprint by Longley et al. to underscore the role that big data plays in the world of infectious diseases. Amassing large amounts of data helps not just in drug discovery, but also in accelerating the development of diagnostic tests that are simultaneously more sensitive and specific, as well as more rapid and inexpensive. This ability to better target antimalarial therapy is especially valuable in reducing toxicities; among these, the most obvious would be to avoid delivering unnecessary and potentially harmful therapies to glucose-6-phosphate deficiency (G6PD) individuals.
The preprint by Sauvey et al. reinforces the value in drug repurposing, and reminds us that even old drugs with considerable side effects can still be useful in the context of other infectious diseases. The authors themselves point out two reasons to be sanguine about mefloquine therapy. First, metronidazole therapy runs the risk of patient noncompliance due to the twin reasons of adverse effects and the need for continued dosing even after symptoms improve; mefloquine’s long half-life may help to target both problems by reducing dosing duration. Second, metronidazole is unable to kill or prevent development of the infective cyst stage of Entamoeba. Interestingly, mefloquine is not fully absorbed in the intestines of patients. While this would be undesirable in other contexts as less of the drug enters systemic circulation, it is fortuitous in the case of amoebiasis, in which the lumen acts as a reservoir for E. histolytica.
Future directions
Future work on malaria will involve expanding the findings made in the two preprints by Longley et al. and Sauvey et al. to other diseases and fields. Longley et al. point out that “similar efforts to develop SEMs are… underway for P. falciparum”, another parasite responsible for malaria. They also point out remaining challenges—one of which is to differentiate between SEMs of malaria-causing parasites, such as that between P. vivax and P. ovale, P. malariae, or P. knowlesi, depending on patterns of co-infection of these other related species.
A similar theme is echoed by Sauvey et al., who investigated mefloquine’s activity against cysts of E. invadens rather than E. histolytica. These interspecies differences will need to be further explored in order to present a better picture of mefloquine’s mechanism of action against E. histolytica. Furthermore, mefloquine’s efficacy will also need to be demonstrated in preclinical animal studies in vivo before progressing to in-human clinical trials.
Exciting times for the field lie ahead. ‘Tis indeed the season to be jolly.
Questions for authors
(A) Longley et al.
- What other developments in characterising the eight shortlisted SEMs will be needed before they can be validated, and a kit can be developed around the shortlisted SEMs?
- Since both the sensitivity and specificity of the model appeared to plateau at about 80%, regardless of the introduction of additional SEMs, what further adjustments can be made to improve the classification algorithm?
(B) Sauvey et al.
- In determining mefloquine and metronidazole activities against mature invadens cysts, how were the concentrations chosen?
- How do the concentrations used in the various assays compare to in vivo concentrations, especially those in the lumen and in the blood?
Sign up to customise the site to your preferences and to receive alerts
Register hereAlso in the microbiology category:
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Microbial Feast or Famine: dietary carbohydrate composition and gut microbiota metabolic function
Jasmine Talevi
Citrobacter rodentium infection activates colonic lamina propria group 2 innate lymphoid cells
André Luiz Amorim Costa, Marcus Oliveira
Also in the pharmacology and toxicology category:
Snake venom metalloproteinases are predominantly responsible for the cytotoxic effects of certain African viper venoms
Daniel Osorno Valencia
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
In vitro pharmacokinetics and pharmacodynamics of the diarylquinoline TBAJ-587 and its metabolites against Mycobacterium tuberculosis
Zhang-He Goh
preLists in the microbiology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |
Also in the pharmacology and toxicology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
COVID-19 / SARS-CoV-2 preprints
List of important preprints dealing with the ongoing coronavirus outbreak. See http://covidpreprints.com for additional resources and timeline, and https://connect.biorxiv.org/relate/content/181 for full list of bioRxiv and medRxiv preprints on this topic
| List by | Dey Lab, Zhang-He Goh |
1
Drug use in special populations
Any drugs that are being used in special populations: Patients with liver and kidney failure, in paediatrics, in geriatrics, and in pregnant or lactating patients. Includes the discovery of factors that could potentially affect drug use in these special populations.
| List by | Zhang-He Goh |
Toxicology of toxicants, existing therapeutics, and investigational drugs
Preprints that describe the toxicology of environmental pollutants and existing and upcoming drugs. Includes both toxicokinetics and toxicodynamics, as well as technological improvements that will help in the characterisation of this field.
| List by | Zhang-He Goh |
Anticancer agents: Discovery and clinical use
Preprints that describe the discovery of anticancer agents and their clinical use. Includes both small molecules and macromolecules like biologics.
| List by | Zhang-He Goh |
Advances in Drug Delivery
Advances in formulation technology or targeted delivery methods that describe or develop the distribution of small molecules or large macromolecules to specific parts of the body.
| List by | Zhang-He Goh |











 (No Ratings Yet)
(No Ratings Yet)