Skd3 (human CLPB) is a potent mitochondrial protein disaggregase that is inactivated by 3-methylglutaconic aciduria-linked mutations
Posted on: 13 February 2020
Preprint posted on 18 January 2020
Article now published in eLife at http://dx.doi.org/10.7554/eLife.55279
Categories: biochemistry
Introduction
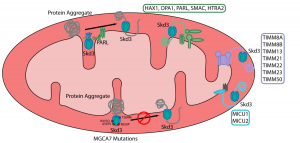
Protein aggregation is a challenge for any living cell, resulting in the loss of protein function and the generation of potentially toxic misfolded protein species. Non-metazoan eukaryotes such as yeast employ specialized molecular chaperones, Hsp104 in the cytosol and nucleus and Hsp78 in mitochondria, that are capable of untangling protein aggregates, allowing the native protein state to be restored. Curiously, these disaggregases seem to have been lost during evolution and are absent in metazoa such as ourselves. Recently, human chaperone complexes capable of disaggregation in the cytosol were identified (1, 2), but a mitochondrial disaggregase so far remained elusive. In this preprint, the authors investigate whether Skd3, an AAA+ ATPase that has some resemblance to Hsp78 and Hsp104, is in fact the long-sought mitochondrial disaggregase in metazoa.
The authors find that Skd3…
- … has ATPase and disaggregase activity. Using in vitro assays, the authors show that purified Skd3 causes aggregated luciferase to partially refold, and that this function is dependent on its ATPase activity. Mutation of a residue in the conserved pore loop that is thought to be critical for substrate binding abolishes the disaggregase activity of Skd3.
- … is regulated by an auto-inhibitory peptide. Skd3 contains a hydrophobic N-terminal stretch that is cleaved by the inner membrane protease PARL. When the authors express Skd3 without this region, mimicking PARL cleavage, they find that the disaggregase activity of Skd3 is boosted by over 10-fold compared to the full-length protein.
- … is capable of disaggregating α-synuclein fibrils. The authors note that the requirements for disaggregating amorphous aggregates versus highly structured amyloid fibrils may differ. However, using a sedimentation assay combined with a dot blot they observe that Skd3 can disaggregate α-synuclein fibrils in the presence of ATP.
- … disease mutations impair the function of the protein. Mutations in Skd3 are associated with the rare mitochondrial disorder 3-methylglutaconic aciduria, type VII (MGCA7). When testing four Skd3 mutants in their in vitro assay, the authors find that the ATPase activities vary but the disaggregase activity correlates with disease severity. Altogether the authors conclude that the function of Skd3 as a mitochondrial disaggregase is crucial for human health.
Why I chose this preprint
As the authors nicely outline in their introduction, it has so far remained a mystery how metazoa can do without the disaggregase proteins Hsp104 and Hsp78 that are present in yeast, but were lost during evolution. Machineries capable of disaggregating amorphous and fibrillar aggregates in the metazoan cytosol have been identified in recent years (1, 2) (see also my recent preLight on this topic), but a mitochondrial disaggregase analogous to yeast Hsp78 had remained elusive. The authors hypothesize that Skd3 could be the long-sought mitochondrial disaggregase, and demonstrate with an elegant series of experiments that this is indeed the case. These findings are disease-relevant, as the authors show that mutations associated with MGCA7 impair the disaggregation function of Skd3. Furthermore, the authors show that Skd3 can disaggregate preformed α-synuclein fibrils, which play a role in Parkinson’s disease and have been suggested to affect mitochondria. Given the importance of mitochondrial protein quality control, the implications of Skd3 activity may well extend more broadly to ageing and neurodegenerative diseases.
Questions
Did the authors test any other candidate mitochondrial disaggregase proteins, or was Skd3 the only obvious choice?
Are the authors aware of a correlation between Skd3 activity and ageing or neurodegenerative diseases?
References
- Nillegoda NB, Kirstein J, Szlachcic A, Berynskyy M, Stank A, Stengel F, Arnsburg K, Gao X, Scior A, Aebersold R, Guilbride DL, Wade RC, Morimoto RI, Mayer MP and Bukau B (2015) Crucial HSP70 co-chaperone complex unlocks metazoan protein disaggregation. Nature 524: 247–251
- Gao X, Carroni M, Nussbaum-Krammer C, Mogk A, Nillegoda NB, Szlachcic A, Guilbride DL, Saibil HR, Mayer MP and Bukau B (2015) Human Hsp70 Disaggregase Reverses Parkinson’s-Linked α-Synuclein Amyloid Fibrils. Mol. Cell 59: 781–793
doi: https://doi.org/10.1242/prelights.16922
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the biochemistry category:
Active flows drive clustering and sorting of membrane components with differential affinity to dynamic actin cytoskeleton
Teodora Piskova
Snake venom metalloproteinases are predominantly responsible for the cytotoxic effects of certain African viper venoms
Daniel Osorno Valencia
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
preLists in the biochemistry category:
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
Peer Review in Biomedical Sciences
Communication of scientific knowledge has changed dramatically in recent decades and the public perception of scientific discoveries depends on the peer review process of articles published in scientific journals. Preprints are key vehicles for the dissemination of scientific discoveries, but they are still not properly recognized by the scientific community since peer review is very limited. On the other hand, peer review is very heterogeneous and a fundamental aspect to improve it is to train young scientists on how to think critically and how to evaluate scientific knowledge in a professional way. Thus, this course aims to: i) train students on how to perform peer review of scientific manuscripts in a professional manner; ii) develop students' critical thinking; iii) contribute to the appreciation of preprints as important vehicles for the dissemination of scientific knowledge without restrictions; iv) contribute to the development of students' curricula, as their opinions will be published and indexed on the preLights platform. The evaluations will be based on qualitative analyses of the oral presentations of preprints in the field of biomedical sciences deposited in the bioRxiv server, of the critical reports written by the students, as well as of the participation of the students during the preprints discussions.
| List by | Marcus Oliveira et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
20th “Genetics Workshops in Hungary”, Szeged (25th, September)
In this annual conference, Hungarian geneticists, biochemists and biotechnologists presented their works. Link: http://group.szbk.u-szeged.hu/minikonf/archive/prg2021.pdf
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |











 (No Ratings Yet)
(No Ratings Yet)