Antifungal effects of PC945, a novel inhaled triazole, on Candida albicans-infected immunocompromised mice
Posted on: 23 September 2020 , updated on: 4 April 2021
Preprint posted on 27 July 2020
To take the yeast by the horns: researchers describe the antifungal effects of a novel inhaled triazole on Candida albicans-infected immunocompromised mice
Selected by Zhang-He GohCategories: microbiology, pharmacology and toxicology
Background of preprint
Candida yeasts are commensal organisms that are often considered as part of the host microbiota. However, they can cause invasive candidiasis in immunocompromised patients, and recent research further suggests that they are also associated with worse clinical outcomes when involved in other respiratory diseases [1-12].
Current antifungal therapy largely revolves around the use of triazoles such as itraconazole, voriconazole, and posaconazole. Unfortunately, clinical antifungal therapy is currently complicated by a few factors: (1) toxicities associated with the antifungals [13,14]; (2) multiple complex drug interactions [15,16]; (3) pharmacokinetic considerations [17]; and (4) global resistance to antimicrobial agents [18]. To circumvent these difficulties, researchers (some of whom also authored this preprint by Nishimoto et al.) invented PC945 [19,20], an antifungal triazole that is designed with improved pharmacokinetics to deliver high local concentrations and for high cell retention (to lengthen its duration of action). In this preprint, Nishimoto et al. evaluated the possibility of administering PC945 intranasally, and assessed its in vitro antifungal effects against various Candida species by comparing it to the positive control voriconazole (Fig. 1).
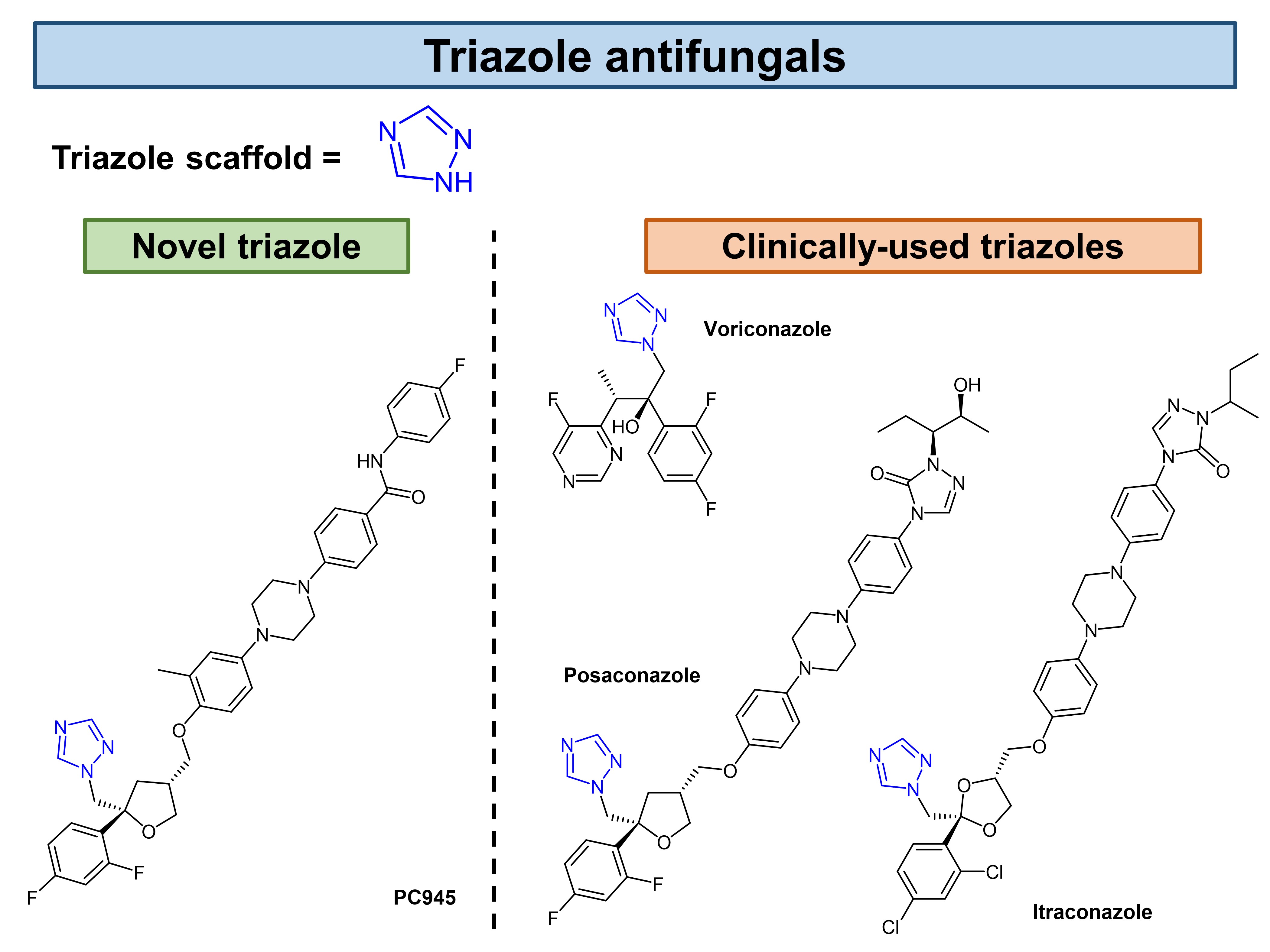
Figure 1. Common antifungals and those investigated by Nishimoto et al. in their preprint.
Key findings of preprint
First, Nishimoto et al. established the survival and biomarkers yardsticks by which to evaluate PC945’s pharmacology. Through these preliminary experiments, the authors established the fungal load inoculum (2.5 x 106) and timeframe (5 days) for their study. They also assessed the in vitro potency of PC945 (0.016 µg/mL) and the positive control voriconazole (0.008 µg/mL).
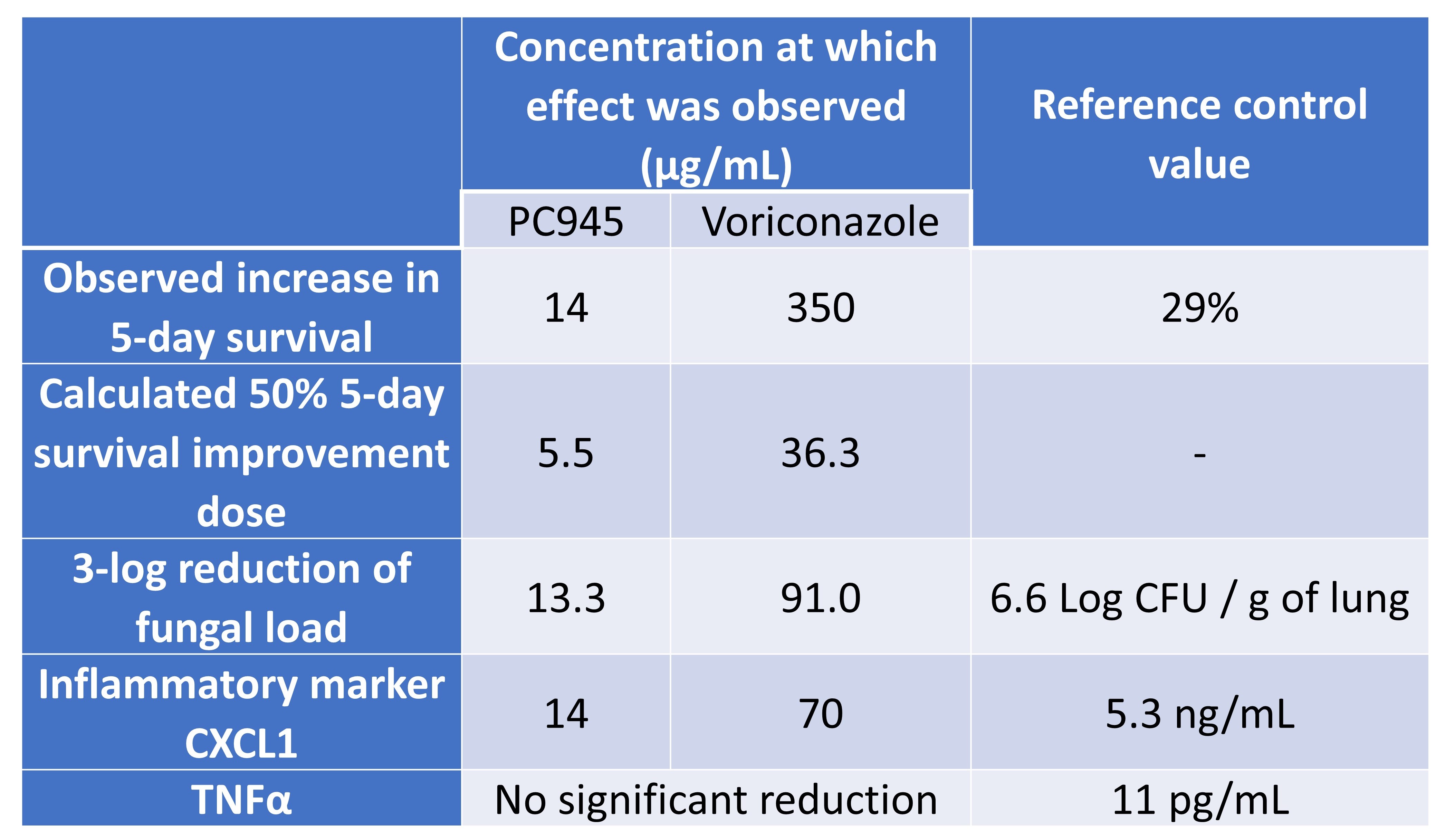
Nishimoto et al. then administered intranasal doses of PC945 or voriconazole in saline suspension to immunocompromised and temporarily neutropaenic mice. They observed a significant, dose-dependent improvement in 5-day survival, fungal burden, and the inflammatory marker CXCL1, but not a significant reduction in TNFα (Table 1). The authors compared these results to another experiment which assessed the efficacy of extended prophylaxis with PC945: prophylaxis was 25-fold more effective than therapeutic treatment in terms of reducing fungal load, and significantly reduced TNFα as well.
Finally, the authors assessed PC945’s in vitro antifungal activity against various Candida species using the Clinical and Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) standards (preprint Table 3, preprint Figure 6). The authors ranked that the antifungals’ potency in the following ascending order: posconzaole < PC945 ≤ voriconazole.
Table 1. Effect of antifungals on mortality, fungal burden, and inflammatory markers.
What I like about this preprint
I selected this preprint because I found it an interesting continuation of the work by the same research group on PC945 in 2017 [19] and 2019 [20], in which Colley et al. discussed the anti-Aspergillus activity of PC945. Previously, Colley et al. characterised the inhibitory activity of PC945 against Aspergillus CYP51 enzymes, as well as the in vitro and in vivo anti-Aspergillus activity of PC945 in various mouse and human alveolar models. In this preprint, Nishimoto et al. apply PC945 to the treatment of fungal infections caused by the Candida species.
Future work
The key questions that remain largely revolve around the factors that account for the vast differences in activity between voriconazole and PC945 (Table 1). Can they be ascribed to the differences in pharmacodynamics, or to the improved pharmacokinetics of PC945? Indeed, in their preprint, Nishimoto et al. highlight the need for further studies to identify the mechanisms involved.
Two main directions lead the way forward. First, further characterisation into the mechanisms underlying PC945’s antifungal activity will allow scientists to better understand its potency, efficacy, toxicity, and selectivity; all these factors are considerations that influence its clinical utility. Second, PC945 may also be investigated for its activity against other fungus species as well, especially those implicated in infectious diseases. In their 2017 paper [19], the authors had found PC945 to be active against Aspergillus, Candida, and Cryptococcus species. Future developments of PC945 may therefore revolve around this line of research.
There is also one other aspect of this research to look forward to. The phase I clinical trial of PC945 was completed in 2018, and the manuscript is in preparation. Nishimoto et al. have suggested in their preprint that the local administration of PC945 largely retains it in the exposed organ—in this case, the lung—with low systemic exposure. Might this pharmacokinetic finding enable clinicians to achieve selectivity through the appropriate route of administration? The pharmacokinetic (PK)-pharmacodynamic (PD) relationship makes for a complex but integral consideration in antimicrobials, so this will be something worth watching.
Open questions
- In your 2017 paper [19], you tested PC945 against many different species of fungi (article Table 6). What made you choose to target the Candida species in this preprint, compared to other fungi?
- What accounts for the observed differences in the potency and efficacy of PC945 against various fungi? Do they arise from differences in pharmacodynamics—for instance, the binding affinity of PC945 may vary across different structural isoforms of CYP51, the putative target? Or do you think there might be pharmacokinetic differences, and species or strains that are more resistant to PC945 could have thicker walls or efflux pumps which reduce penetration of PC945 into these fungi?
References
[1] van der Geest PJ, Dieters EI, Rijnders B, Groeneveld JAB, Safety and efficacy of amphotericin-B deoxycholate inhalation in critically ill patients with respiratory Candida spp. colonization: a retrospective analysis, BMC Infectious Diseases 14(1) (2014) 575.
[2] Barchiesi F, Orsetti E, Gesuita R, Skrami E, Manso E, Epidemiology, clinical characteristics, and outcome of candidemia in a tertiary referral center in Italy from 2010 to 2014, Infection 44(2) (2016) 205-213.
[3] Máiz L, Girón R, Olveira C, Vendrell M, Nieto R, Martínez-García MA, Prevalence and factors associated with nontuberculous mycobacteria in non-cystic fibrosis bronchiectasis: a multicenter observational study, BMC Infect Dis 16(1) (2016) 437.
[4] O’Driscoll BR, Hopkinson LC, Denning DW, Mold sensitization is common amongst patients with severe asthma requiring multiple hospital admissions, BMC Pulm Med 5 (2005) 4.
[5] Chowdhary A, Agarwal K, Kathuria S, Gaur SN, Randhawa HS, Meis JF, Allergic bronchopulmonary mycosis due to fungi other than Aspergillus: a global overview, Critical Reviews in Microbiology 40(1) (2014) 30-48.
[6] Mullaoglu S, Turktas H, Kokturk N, Tuncer C, Kalkanci A, Kustimur S, Esophageal candidiasis and Candida colonization in asthma patients on inhaled steroids, Allergy Asthma Proc 28(5) (2007) 544-549.
[7] Ramachandran S, Shah A, Pant K, Bhagat R, Jaggi OP, Allergic bronchopulmonary aspergillosis and Candida albicans colonization of the respiratory tract in corticosteroid-dependent asthma, Asian Pac J Allergy Immunol 8(2) (1990) 123-126.
[8] Dermawan JKT, Ghosh S, Keating MK, Gopalakrishna KV, Mukhopadhyay S, Candida pneumonia with severe clinical course, recovery with antifungal therapy and unusual pathologic findings: A case report, Medicine (Baltimore) 97(2) (2018) e9650.
[9] Yazici O, Cortuk M, Casim H, Cetinkaya E, Mert A, Benli AR, Candida glabrata Pneumonia in a Patient with Chronic Obstructive Pulmonary Disease, Case Rep Infect Dis 2016 (2016) 4737321.
[10] Nguyen LDN, Viscogliosi E, Delhaes L, The lung mycobiome: an emerging field of the human respiratory microbiome, Frontiers in microbiology 6 (2015) 89-89.
[11] Máiz L, Nieto R, Cantón R, Gómez GdlPE, Martinez-García M, Fungi in Bronchiectasis: A Concise Review, Int J Mol Sci 19(1) (2018).
[12] Johnson DC, Chronic candidal bronchitis: a consecutive series, Open Respir Med J 6 (2012) 145-149.
[13] Xiong W-H, Brown RL, Reed B, Burke NS, Duvoisin RM, Morgans CW, Voriconazole, an Antifungal Triazol That Causes Visual Side Effects, Is an Inhibitor of TRPM1 and TRPM3 Channels, Investigative Ophthalmology & Visual Science 56(2) (2015) 1367-1373.
[14] Thompson GR, 3rd, Lewis JS, 2nd, Pharmacology and clinical use of voriconazole, Expert Opin Drug Metab Toxicol 6(1) (2010) 83-94.
[15] Brüggemann RJ, Donnelly JP, Aarnoutse RE, Warris A, Blijlevens NM, Mouton JW, Verweij PE, Burger DM, Therapeutic drug monitoring of voriconazole, Ther Drug Monit 30(4) (2008) 403-411.
[16] Jeong S, Nguyen PD, Desta Z, Comprehensive in vitro analysis of voriconazole inhibition of eight cytochrome P450 (CYP) enzymes: major effect on CYPs 2B6, 2C9, 2C19, and 3A, Antimicrob Agents Chemother 53(2) (2009) 541-551.
[17] Rodvold KA, George JM, Yoo L, Penetration of anti-infective agents into pulmonary epithelial lining fluid: focus on antibacterial agents, Clin Pharmacokinet 50(10) (2011) 637-664.
[18] Drusano GL, Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug’, Nature Reviews Microbiology 2(4) (2004) 289-300.
[19] Colley T, Alanio A, Kelly SL, Sehra G, Kizawa Y, Warrilow AGS, Parker JE, Kelly DE, Kimura G, Anderson-Dring L, Nakaoki T, Sunose M, Onions S, Crepin D, Lagasse F, Crittall M, Shannon J, Cooke M, Bretagne S, King-Underwood J, Murray J, Ito K, Strong P, Rapeport G, In Vitro and In Vivo Antifungal Profile of a Novel and Long-Acting Inhaled Azole, PC945, on Aspergillus fumigatus Infection, Antimicrobial agents and chemotherapy 61(5) (2017) e02280-02216.
[20] Colley T, Sehra G, Daly L, Kimura G, Nakaoki T, Nishimoto Y, Kizawa Y, Strong P, Rapeport G, Ito K, Antifungal synergy of a topical triazole, PC945, with a systemic triazole against respiratory Aspergillus fumigatus infection, Scientific Reports 9(1) (2019) 9482.
doi: https://doi.org/10.1242/prelights.24507
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the microbiology category:
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Microbial Feast or Famine: dietary carbohydrate composition and gut microbiota metabolic function
Jasmine Talevi
Citrobacter rodentium infection activates colonic lamina propria group 2 innate lymphoid cells
André Luiz Amorim Costa, Marcus Oliveira
Also in the pharmacology and toxicology category:
Snake venom metalloproteinases are predominantly responsible for the cytotoxic effects of certain African viper venoms
Daniel Osorno Valencia
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
In vitro pharmacokinetics and pharmacodynamics of the diarylquinoline TBAJ-587 and its metabolites against Mycobacterium tuberculosis
Zhang-He Goh
preLists in the microbiology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |
Also in the pharmacology and toxicology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
COVID-19 / SARS-CoV-2 preprints
List of important preprints dealing with the ongoing coronavirus outbreak. See http://covidpreprints.com for additional resources and timeline, and https://connect.biorxiv.org/relate/content/181 for full list of bioRxiv and medRxiv preprints on this topic
| List by | Dey Lab, Zhang-He Goh |
1
Drug use in special populations
Any drugs that are being used in special populations: Patients with liver and kidney failure, in paediatrics, in geriatrics, and in pregnant or lactating patients. Includes the discovery of factors that could potentially affect drug use in these special populations.
| List by | Zhang-He Goh |
Toxicology of toxicants, existing therapeutics, and investigational drugs
Preprints that describe the toxicology of environmental pollutants and existing and upcoming drugs. Includes both toxicokinetics and toxicodynamics, as well as technological improvements that will help in the characterisation of this field.
| List by | Zhang-He Goh |
Anticancer agents: Discovery and clinical use
Preprints that describe the discovery of anticancer agents and their clinical use. Includes both small molecules and macromolecules like biologics.
| List by | Zhang-He Goh |
Advances in Drug Delivery
Advances in formulation technology or targeted delivery methods that describe or develop the distribution of small molecules or large macromolecules to specific parts of the body.
| List by | Zhang-He Goh |











 (No Ratings Yet)
(No Ratings Yet)