Engineered Nanotopographies Induce Transient Openings in the Nuclear Membrane
Posted on: 23 September 2024 , updated on: 3 October 2024
Preprint posted on 16 August 2024
Article now published in at https://onlinelibrary.wiley.com/doi/epdf/10.1002/adfm.202410035
Categories: bioengineering, biophysics
Updated 23 September 2024 with a postLight by Sristilekha Nath
This preprint has now been published in Advanced Functional Materials with minor revisions. Notably, a figure showing the confocal image of nano-pillars sitting on cells was added to the published version. I believe this addition helps readers better visualize the actual experimental setup. It is interesting that the publication continues to portray its novel findings, presenting it as a promising approach for potential use in clinical applications.
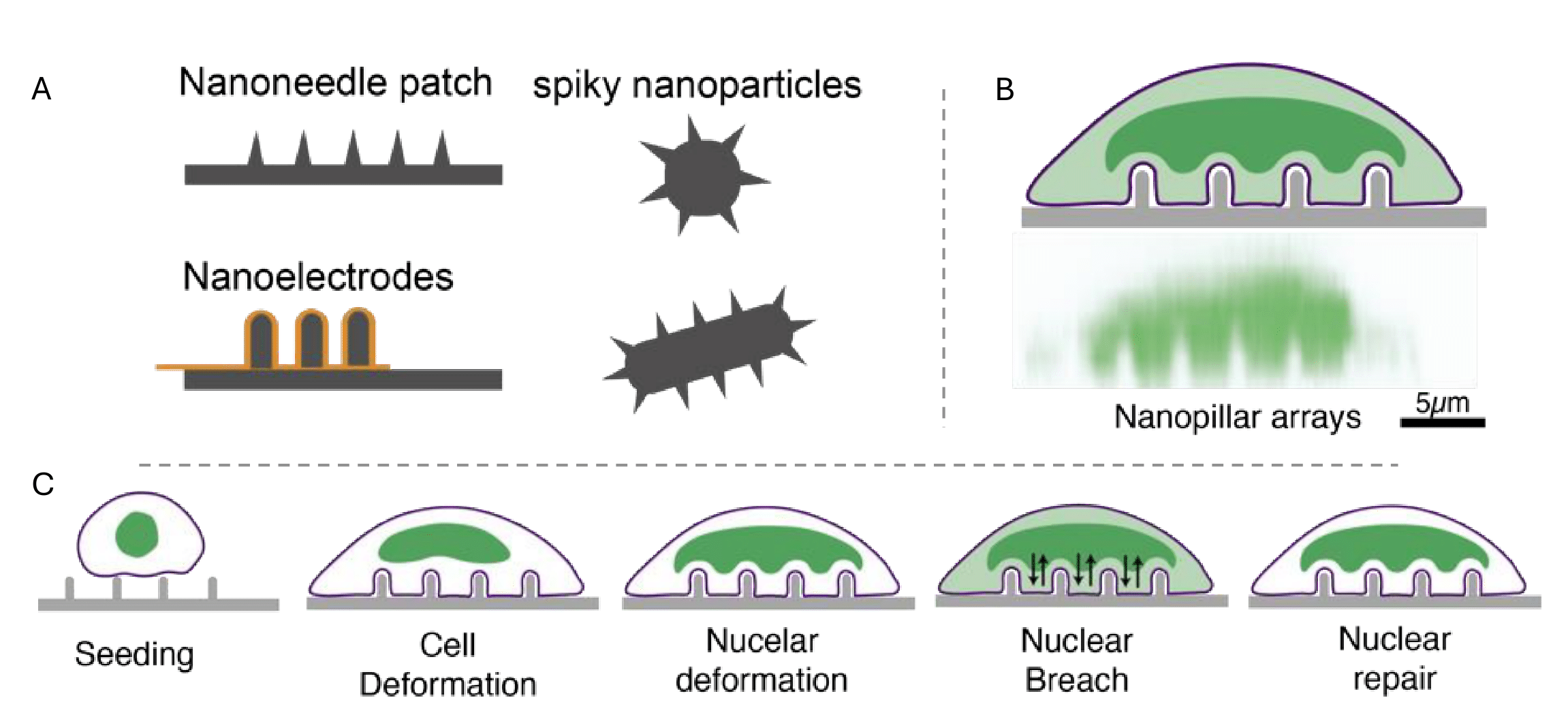
Background
Recent technological advancements have enabled the creation of diverse nanotopographical structures that mimic naturally occurring living particles such as viral spike proteins. Like these natural particles, engineered materials can respond to physical interactions with cells by eliciting specific signaling pathways and cell behaviors. Previously reported responses included cytoskeletal remodeling, plasma membrane curvature modulation, penetration, and endocytosis, which can enhance functions like cellular uptake during drug delivery. However, the effects of nanomaterial interactions with other cellular organelles remain poorly understood. To address this gap, the authors of this preprint examine nuclear responses as well as changes in its morphology and behavior elicited by physical interactions between engineered materials like nanopillars and different cell types. This may offer valuable insights for the design of targeted clinical interventions.
Key findings
Nanoscale curvature induced by nanotopographies triggers nuclear membrane breaches across different cell types
Previous studies show that nanostructures in contact with a cell can alter nuclear membrane curvature: both positive and negative curvatures and cause nuclear rupture. The authors therefore wondered if the deformed curvature induced by nanopillars could breach the nuclear membrane. To verify this, they stained cells on engineered nanopillars with a nuclear rupture reporter, known to be present in the nucleus of a normal cell and to diffuse to the cytoplasm upon a breach in the nucleus. Testing cells with diverse structural and morphological features, they found that the nuclear rupture reporter was detectable in the cytoplasm of cells sitting on nanopillars but not those on flat substrates. This confirmed the creation of nuclear membrane openings by the nanopillar substrates. Interestingly, the authors could also observe an opposite diffusion process in cells sitting on nanopillars: from the cytoplasm to the nucleus. These observations led to the conclusion that nanopillar-cell interactions can facilitate bidirectional movement of molecules between cytoplasm and nucleus by inducing nuclear breaches.
Duration of nanopillar-cell interaction and nanopillar geometry control nuclear membrane breaches
Next, the authors speculated that the nuclear breaches might be dependent on the time of contact between the nanopillars and the cells. They found that the observed nuclear openings indeed depend on the duration of this interaction. They also revealed that while the nucleus was gradually deformed (by increasing its curvature within 1 to 8 hours), it was only during the initial 1 to 5 hours that the nuclear breaches most evidently increased. Quantification of other morphological features, such as cell and nuclear circularity and spreading area under induced breaching conditions, showed that rather than cell dimensions, nuclear dimensions like spreading, and deformation are key drivers of nuclear membrane breaching on nanopillars. In addition, nanopillars of small size increased nuclear curvature, which was critical for nuclear membrane breaching phenomena.
Induced nuclear membrane openings are transient and repairable
Live imaging experiments demonstrated that the nuclear membrane, temporarily breached by the engineered nanotopographies enabling molecular exchange with the cytoplasm, is capable of self-repair. They confirmed this by expressing a nuclear rupture reporter: its cytoplasmic to nuclear ratio gradually decreased within 1.5 hours, indicating that the repair mechanism might be activated approximately within that period post-breaching. Further investigation uncovered the recruitment of a tissue-resident repair machinery, ESCRT-III, to the nuclear membrane opening sites which aided the repair process.
Why I highlight this preprint
The author’s straightforward, yet insightful approach to investigate how diverse shape and sizes of nanomaterials can affect nuclear responses is noteworthy. Evidently, nuclear membrane pores play a key role in facilitating molecular exchanges between the cytoplasm and nucleus, influencing signaling cascades crucial for cell survival and growth. Looking at the broader picture, these pathways ultimately regulate tissue development. Therefore, having control over the nuclear pores or openings -allowing more molecular exchanges and interactions without causing damage to the cells – may possibly enhance cellular mechanisms as desired. Especially the results that showed how engineered nanomaterials can create temporary openings in the nucleus, which can also be partially reversed, caught my attention. By employing a simple trick to glimpse into the nucleus and allowing exchange of essential molecular information, this study presents a promising and effective strategy for drug delivery and other targeted therapies that rely on controlled interactions while minimizing damage.
Questions for the authors
- During the transient openings in the nuclear membrane, do the authors believe that there might be an uncontrolled exchange of signaling molecules between the cytoplasm and nucleus, which might affect other active cellular processes? In that case, is there any way to maintain a proper balance of such molecular exchanges during the transient breaching period?
- Is there a possibility that nanotopographic materials of diverse geometry can elicit undesired host immune response whenever applied for therapeutic purposes?
References
Hansel, C.S. et al. (2019) ‘Nanoneedle-mediated stimulation of cell mechanotransduction machinery’, ACS Nano, 13(3), pp. 2913–2926. doi:10.1021/acsnano.8b06998.
Sarikhani, E. et al. (2024) ‘Engineering the cellular microenvironment: Integrating three-dimensional nontopographical and two-dimensional biochemical cues for precise control of cellular behavior’, ACS Nano, 18(29), pp. 19064–19076. doi:10.1021/acsnano.4c03743.
Chiappini, C. et al. (2021) ‘Tutorial: Using nanoneedles for Intracellular Delivery’, Nature Protocols, 16(10), pp. 4539–4563. doi:10.1038/s41596-021-00600-7.
doi: https://doi.org/10.1242/prelights.38439
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the bioengineering category:
Matrix viscoelasticity regulates dendritic cell migration and immune priming
Roberto Amadio
Human pluripotent stem cell-derived atrioventricular node-like pacemaker cells exhibit biological conduction bridge properties in vitro and in vivo
Theodora Stougiannou
Capillary constrictions prime cancer cell tumorigenicity through PIEZO1
Sristilekha Nath
Also in the biophysics category:
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
Mitochondrial Fatty Acid Oxidation is Stimulated by Red Light Irradiation
Rickson Ribeiro, Marcus Oliveira
ROCK2 inhibition has a dual role in reducing ECM remodelling and cell growth, while impairing migration and invasion
Sharvari Pitke
preLists in the bioengineering category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Advances in microscopy
This preList highlights exciting unpublished preprint articles describing advances in microscopy with a focus on light-sheet microscopy.
| List by | Stephan Daetwyler |
Also in the biophysics category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
66th Biophysical Society Annual Meeting, 2022
Preprints presented at the 66th BPS Annual Meeting, Feb 19 - 23, 2022 (The below list is not exhaustive and the preprints are listed in no particular order.)
| List by | Soni Mohapatra |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
Biophysical Society Meeting 2020
Some preprints presented at the Biophysical Society Meeting 2020 in San Diego, USA.
| List by | Tessa Sinnige |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Biomolecular NMR
Preprints related to the application and development of biomolecular NMR spectroscopy
| List by | Reid Alderson |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |











 (No Ratings Yet)
(No Ratings Yet)