Sex-dependent resource defense in a nectar-feeding bat
Posted on: 1 October 2021 , updated on: 2 October 2021
Preprint posted on 17 August 2021
The furry fight for flowers: Male long-tongued bats defend flowers from other males and show sex-specific territoriality during nectar feeding
Selected by Baheerathan MurugavelCategories: animal behavior and cognition, bioengineering, ecology, zoology
Background
Animals compete for resources under resource-limited situations. Within-species competition can happen either directly or indirectly, depending upon how individuals interact with their environment. Interference competition occurs when an individual defends resources and directly reduces access of resources to others. For example, nectar-feeding birds establish and defend long-term feeding territories, but such territories are not known in the neotropical nectar feeding bats over an extended time period. However, there is evidence of aggressive food defense at floral inflorescence by several species of bats in the nectar feeding genus Glossophaga1,2. Optimality models3,4 predict that feeding territoriality is defined by the levels of food abundance. According to these models, resource defense is predicted to occur most commonly at intermediate levels of food abundance, as low abundance could not meet the energetic costs of resource defense, and high resource abundance would be too difficult for an individual to defend alone.
In this preprint, the authors tested these predictions in the flower visiting bat Glossophaga mutica under lab conditions. The authors established an experimental design mimicking naturalistic foraging by creating computer-controlled artificial flowers. Equipped with ID sensors, these flowers electronically identified an ID-tagged bat during flower visits, and their nectar levels were programmed to be monitored and replenished, as in the natural flowers. Using this design and supporting video observations, the authors investigated resource defense behavior and the effect of resource abundance on territoriality within and among sexes
Key findings
Using 36 individuals of G. mutica, the authors created six experimental groups of six bats each. Four of these were mixed-sex groups and the remaining two were all male or all female groups. A set of 10 equally spaced artificial flowers were housed in two patches of five each and covered with a plastic sheet. During a training phase (one to four nights), the plastic sheets were open and all of the bats were trained to visit and feed on the artificial flowers in both the patches. Training was considered to be complete once the bats started visiting the flowers regularly. During the experimental phase, the plastic sheets were rolled down leaving a narrow opening at the bottom which acted as an entrance for the bats. Thus, during experiments a bat should enter the opening and fly upwards to feed on the flowers. In the first half of the experimental nights (phase 1), only one patch of flowers was rewarded. In the next half, both the patches were rewarded (phase 2), thus mimicking clumped and distributed resource conditions. Nectar intake and the ID of the bat visiting were recorded using the computerized flowers. Individual behavior was recorded by additional video observations at both the patches.
The main observations by the authors were:
- Nectar consumption varied between sexes and individuals where the males seemed to monopolize the flowers during the clumped resource situation. Males chased other bats more frequently than females and the bats that were chased away had lower rates of nectar consumption.
- Comparing nectar intake and chasing events among males, it was clear that certain males in each group were ‘dominant’ by chasing others and defending the flowers. Out of all the bats, the authors report six such dominant males (one from each mixed-sex group and two from the all-male group) with the rest being ‘subordinate’ males (Figure).
- In the female-only group there were cases of four females chasing others, of which one seemed to be relatively more dominant over the others (Figure). However, females did not show any such aggression when males were present.
- Video observations revealed behaviors of male dominance. For instance, some males were seen hanging between the flowers for a significant amount of time and threatened a visiting individual by opening its wings. There were also cases where these dominant males chased away the intruders by following them for short distances. Although it was not possible to ID these bats in the videos, the authors report cases of fresh scratches on the wing of a subordinate male that could have been resulted from bites from a dominant male.
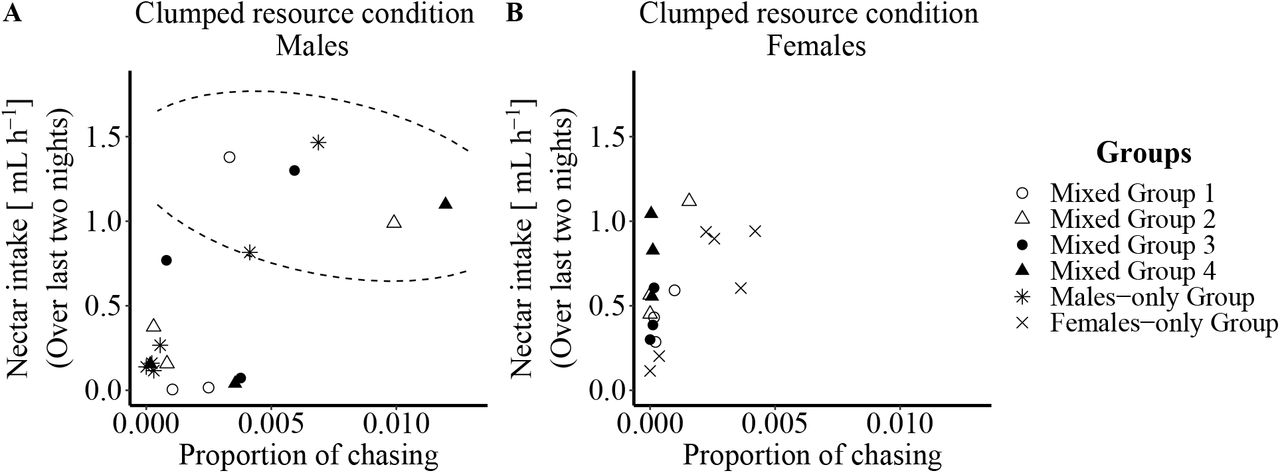
Figure (from the preprint): Influence of chasing frequency on nectar intake in the clumped resource condition during the last two nights of the experiment. (A) Males that more often chased other males also consumed more nectar. Males were divided into two non-overlapping groups by considering the chasing frequency and the amount of nectar an individual received during the clumped resource condition at the end of the experiment. Dominant males (inside dashed line oval) met two criteria: they chased other individuals at flowers more frequently (>0.003) and received more nectar (>0.75mL h−1) during the clumped resource condition. Individuals outside the dashed line oval were categorized as subordinate males. (B) Nectar consumption of females did not generally depend on chasing frequency during the clumped resource condition and non-overlapping groups did not emerge (Credits: authors of the preprint).
Thus, this study describes the existence of a sex-dependent resource defense in G. mutica. As the frequency of aggression was higher in the clumped resource condition, these findings support the model predictions. Even though females were chased away by the dominant males, nectar intake was not affected in females as in the subordinate males. Also, females tried to defend flowers only in the absence of males. Thus, the authors discuss the involvement of a social hierarchy in resource defense in this species. Finally, the authors compare their findings with field observations in other Glossophaga species1 and discuss the implications of their findings in understanding resource defense behavior in free-living nectar feeding bats.
Why I chose this preprint
Studying foraging ecology in nectar-feeding bats has always been challenging considering their body size, behavior and foraging heights. Due to these reasons, there have been relatively few studies describing foraging behaviors or exploring spatial and temporal patterns of foraging in free-ranging flower visiting bats. Documentation of foraging behaviors linked to resource competition in a nectar-feeding bat species has potential implications for understanding the behavioral ecology and costs associated with competition in plant-visiting bats. Adapting existing methods, this study has used a laboratory based experimental design to document sex-dependent resource defense behavior in the nectar-feeding long tongued bat Glossophaga mutica. The authors have not only described but quantified and compared resource defense behaviors among individuals in their study. These methods can potentially help designing future experiments on understanding foraging behaviors in similar sized flower visiting bats.
Questions to the authors
- Do you expect similar results if the experimental nights were split into four phases (~3h each) with alternating conditions of clumped and uniform resource conditions? Is there a reason for splitting the nights into just two phases?
- Why was the duration of the first half variable in the same sex groups and not in the mixed-sex groups?
- How was the cut-off for the two groups (dominant and subordinate) of males chosen? What was the criteria for fixing >0.003 as a proportion of dominance? I ask this because there is a bat from the mixed group 3 that has received more nectar (~0.75 mL h-1) but has been grouped as a sub-ordinate due to the chasing frequency. Could you explain this criterion more?
- How visible were the plastic split rings in the videos? Can those forearm markings be used to identify individuals in the videos? If not, can any other markings (e.g. color codes or reflector tapes) be useful for detections in videos?
References
- Lemke, T. O. Foraging Ecology of the Long-Nosed Bat, Glossophaga soricina, With Respect to Resource Availability. Ecology 65, 538–548 (1984).
- Tschapka, M. Pollination of the understorey palm Calyptrogyne ghiesbreghtiana by hovering and perching bats. Biol. J. Linn. Soc. 80, 281–288 (2003).
- Grant, J. W. A., Girard, I. L., Breau, C. & Weir, L. K. Influence of food abundance on competitive aggression in juvenile convict cichlids. Anim. Behav. 63, 323–330 (2002).
- Grant, J. W. A. Whether or not to defend? The influence of resource distribution. Mar. Behav. Physiol. 23, 137–153 (1993)
doi: https://doi.org/10.1242/prelights.30771
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the animal behavior and cognition category:
Cannibalism as a mechanism to offset reproductive costs in three-spined sticklebacks
Tina Nguyen
Morphological variations in external genitalia do not explain the interspecific reproductive isolation in Nasonia species complex (Hymenoptera: Pteromalidae)
Stefan Friedrich Wirth
Trade-offs between surviving and thriving: A careful balance of physiological limitations and reproductive effort under thermal stress
Tshepiso Majelantle
Also in the bioengineering category:
A Novel Chimeric Antigen Receptor (CAR) - Strategy to Target EGFRVIII-Mutated Glioblastoma Cells via Macrophages
Dina Kabbara
Human pluripotent stem cell-derived macrophages modify development of human kidney organoids
Theodora Stougiannou
Matrix viscoelasticity regulates dendritic cell migration and immune priming
Roberto Amadio
Also in the ecology category:
Resilience to cardiac aging in Greenland shark Somniosus microcephalus
Theodora Stougiannou
Cannibalism as a mechanism to offset reproductive costs in three-spined sticklebacks
Tina Nguyen
Trade-offs between surviving and thriving: A careful balance of physiological limitations and reproductive effort under thermal stress
Tshepiso Majelantle
Also in the zoology category:
Resilience to cardiac aging in Greenland shark Somniosus microcephalus
Theodora Stougiannou
DNA Specimen Preservation using DESS and DNA Extraction in Museum Collections: A Case Study Report
Daniel Fernando Reyes Enríquez, Marcus Oliveira
Morphological variations in external genitalia do not explain the interspecific reproductive isolation in Nasonia species complex (Hymenoptera: Pteromalidae)
Stefan Friedrich Wirth
preLists in the animal behavior and cognition category:
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Bats
A list of preprints dealing with the ecology, evolution and behavior of bats
| List by | Baheerathan Murugavel |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Also in the bioengineering category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Advances in microscopy
This preList highlights exciting unpublished preprint articles describing advances in microscopy with a focus on light-sheet microscopy.
| List by | Stephan Daetwyler |
Also in the ecology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
EMBO | EMBL Symposium: The organism and its environment
This preList contains preprints discussed during the 'EMBO | EMBL Symposium: The organism and its environment', organised at EMBL Heidelberg, Germany (May 2023).
| List by | Girish Kale |
Bats
A list of preprints dealing with the ecology, evolution and behavior of bats
| List by | Baheerathan Murugavel |
Also in the zoology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |











 (No Ratings Yet)
(No Ratings Yet)