The Wnt pathway scaffold protein Axin promotes signaling specificity by suppressing competing kinase reactions
Posted on: 17 September 2019 , updated on: 18 September 2019
Article now published in Cell Systems at http://dx.doi.org/10.1016/j.cels.2020.05.002
Axin, a Wnt pathway scaffold protein, promotes GSK3β affinity for β-catenin and suppresses GSK3β affinity for competing substrates
Selected by Yasmin LauCategories: biochemistry
Background
GSK3β is a kinase involved in a variety of different signalling pathways which are activated in response to a range of cues and is conserved from yeast to mammals (1). It is a key player in pathways such as the Hedgehog pathway, Wnt signalling, as well as G-protein coupled receptor-dependent pathways. Specifically, activity of the Wnt pathway is crucial in determining cell fate, polarity and migration (2). Its disruption has also been implicated to be tightly associated with tumour development (3). As such, the dissection of the biochemical mechanisms, activities and regulation of such versatile signalling proteins is vital.
β-catenin is a component of the Wnt pathway which is phosphorylated by GSK3β and subsequently degraded. The scaffold protein Axin mediates the assembly of the GSK3β-β-catenin complex, and concomitantly it is hypothesized that Axin promotes GSK3β-dependent phosphorylation of β-catenin in the absence of a Wnt signal (4)(5). β-catenin phosphorylation requires CK1, GSK3β and Axin as a “destruction complex”. However, the molecular mechanism by which Wnt signals regulate β-catenin phosphorylation and degradation is unclear. Opposingly, β-catenin phosphorylation is blocked in response to a Wnt signal, whereby β-catenin accumulates and stimulates downstream gene expression (Figure 1).
Figure 1:
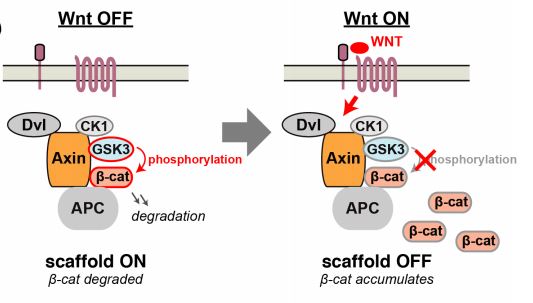
In this preprint, the authors aim to elucidate the mechanisms by which Wnt blocks β-catenin phosphorylation via Axin. This is achieved by asking whether Axin promotes GSK3β phosphorylation of β-catenin, which was tested by measuring the rate of reactions catalyzed by GSK3β with and without Axin in vitro.
Key Findings
Measuring binding affinities and rate constants of recombinant Axin with GSK3β and recombinant β-catenin
The authors first purified GSK3β from E. coli and also purified a recombinant phospho-Ser45-β-catenin. A full-length and mini Axin (mini fragment of the full-length), both containing GSK3β and β-catenin binding domains, were also purified. Subsequently, the binding affinities of both Axin and mini Axin towards GSK3β and β-catenin were measured with bio-layer interferometry, where Kd of the full-length Axin and mini Axin were 7.5 nM and 16 nM respectively. The Kd of the mini Axin for the recombinant primer β-catenin was 4 µM. These values are similar to previously published values of Axin binding affinity for human GSK3β (65 nM) and unprimed mouse β-catenin (1.6 µM), demonstrating that the recombinant Axin binds to both GSK3β and the recombinant β-catenin in vitro.
Next, the phosphorylation rates of β-catenin with and without mini Axin were measured using Western blotting, as it is specifically phosphorylated at sites T41, S37 and S33 (6). Thus, antibodies specific for these sites were used to quantify phosphorylated β-catenin. The Kcat was increased by 1.5-fold and Km was decreased by 2-fold in the presence of mini Axin, while Kcat/Km was increased by 3-fold (Figure 2b & 2c). the 2-fold decrease in Km shows that the mini Axin promotes GSK3β and β-catenin binding to some extent; however, these values are much lower than those previously published (7).
Figure 2:
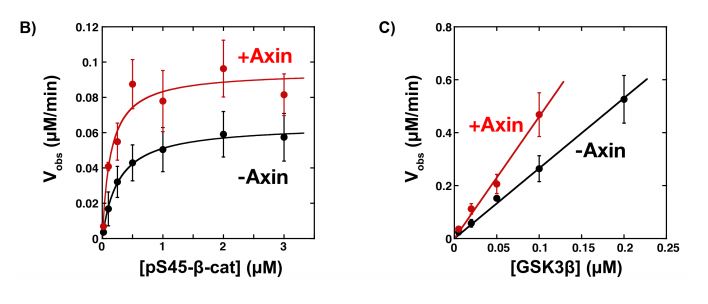
The β-catenin binding site on Axin is necessary for the Axin-GSK3β complex activity
Because the binding affinity of Axin for β-catenin was significantly weaker than that for GSK3β and it was previously demonstrated that the Kd of GSK3β for β-catenin was similar to that of the Axin-GSK3β complex, it was tested whether the β-catenin binding site of Axin was dispensible. Here, the BCD (β-catenin binding domain) of Axin was removed (Figure 3a) and its effects on GSK3β activity were subsequently tested using the same kinetic system. Compared to the presence of free GSK3β, the rate of phosphorylation of β-catenin in the presence of the Axin∆BCD-GSK3β complex appeared to be significantly lower (Figure 3b). Interestingly, free GSK3β is structurally very similar to GSK3β bound to Axin∆BCD. Moreover, this observation is reversed and the phosphorylation rate is restored when Axin is able to bind β-catenin. This indicates that somehow, the BCD of Axin is required for mediating GSK3β binding to β-catenin.
Figure 3:
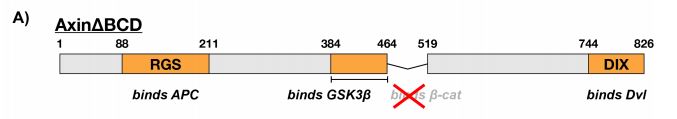
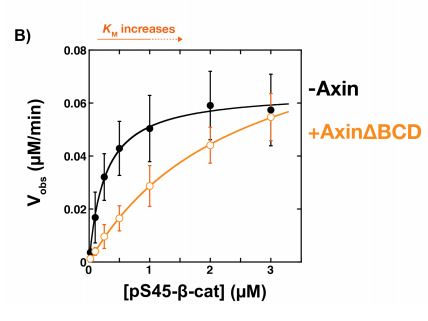
Axin suppresses GSK3β activity with CREB, a non-Wnt pathway substrate
Since Axin has the ability to weaken GSK3β binding to its substrates as well as promote β-catenin binding, it was investigated whether this was also the case with non-wnt signaling substrates. The reaction rates of GSK3β with CREB, another substrate of GSK3β, were measured using a short phosphorylated primed CREB peptide. It was found that the presence of Axin caused a 20-fold decrease in phosphorylation rate of CREB. This suggests that binding of Axin lacking a CREB binding site to GSK3β reduces CREB substrate binding.
Unprimed β-catenin leads to an accumulation of an inactive β-catenin-GSK3β oligomer
Following up the rate of phosphorylation in the presence and absence of Axin, it was asked why the 2-fold increase in rate with Axin was significantly lower than that in previous studies. This could possibly be due to the fact that these previous studies were performed with unprimed β-catenin, which means β-catenin has not been phosphoprimed (on Ser45) (8)(9). Consequently, this suggests that Axin may promote GSK3β binding to β-catenin via phosphopriming. Thus, additional experiments were performed with unprimed β-catenin.
It was found that while the unprimed reaction rates were 100 fold slower than when primed, the rate constants were not effected much by Axin. Moreover, without Axin, when the concentrations of GSK3β were varied with unprimed β-catenin, the rate increases steadily but levels off at 100 nM (Figure 4b), while in the presence of Axin the rate increases linearly (Figure 4a). It was suggested that in the absence of Axin, an inactive GSK3β-β-catenin dimer or oligomer is formed which leads to the plateau in rate, and somehow Axin prevents this formation.
Figure 4:
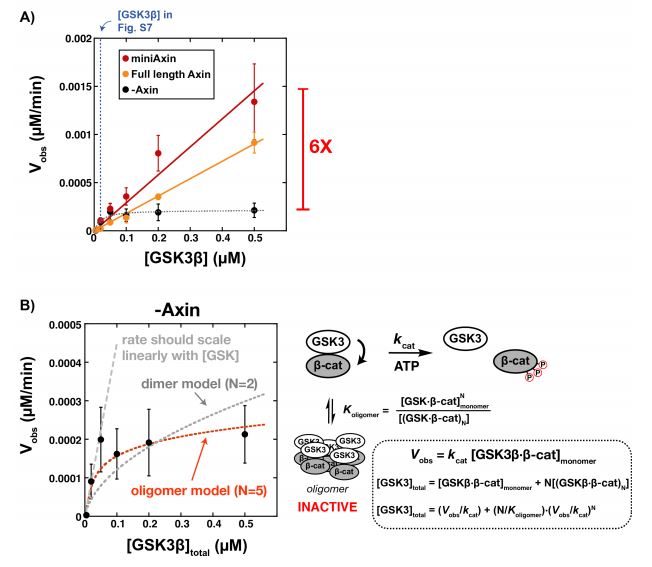
Interestingly, the inactive state of GSK3β-β-catenin is only formed in the presence of unprimed β-catenin, as this plateau was only observed in this experiment, but not with GSK3β and phospho-Ser45-β-catenin.
Why I like this preprint
While this preprint focuses on a very specific functional mechanism of GSK3β and a small portion of the Wnt signaling pathway, it provides further insight into the possible behaviour of GSK3β in other signalling pathways based on its structure. We also learn that it has unexpected requirements of a scaffold protein for its functionality. Moreover, similar techniques with a kinetic model used in this study can also be transferred to elucidate many other mechanisms of GSK3β within other pathways to measure binding affinities and reaction rates catalyzed by GSK3β , perhaps mostly involving GSK3β-dependent phosphorylation.
Questions
- Since Axin lacking the BCD plus GSK3β was used in testing rates of phosphorylation against free GSK3β, was the wild-type Axin also tested with GSK3β against free GSK3β in terms of pS45-β-catenin binding? Would the rate of reaction expected to be even higher than that of in the presence of free GSK3β?
- Are there any other known mechanisms in which GSK3β plays a major role which are similar to that of the Axin-GSK3β-β-catenin reaction? Also, are there any other cases where GSK3β has been found to form inactive oligomers with its substrate?
References
1. Wu D, Pan W (2010) GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci 35(3): 161-168.
2. Komiya Y, Habas R (2008) Wnt signal transduction pathways. Organogenesis 4(2): 68-75.
3. Zhan T, Rindtorff N, Boutros M (2017) Wnt signaling in cancer. Oncogene 36: 1461-1473.
4. Polakis P (2000) Wnt signaling and cancer. Genes Dev 14(15):1837–1851.
5. Moon RT, Kohn AD, De Ferrari GV, Kaykas A (2004) WNT and β-catenin signalling:
diseases and therapies. Nat Rev Genet 5(9):691–701.
6. Liu C, et al. (2002) Control of β-catenin phosphorylation/degradation by a dual-kinase
mechanism. Cell 108(6):837–847.
7. Ikeda S, et al. (1998) Axin, a negative regulator of the Wnt signaling pathway, forms a
complex with GSK-3β and β-catenin and promotes GSK-3β-dependent phosphorylation of
β-catenin. EMBO J 17(5):1371–1384.
8. Amit S, et al. (2002) Axin-mediated CKI phosphorylation of β-catenin at Ser 45: a
molecular switch for the Wnt pathway. Genes Dev 16(9):1066–1076.
9. Liu C, et al. (2002) Control of β-catenin phosphorylation/degradation by a dual-kinase
mechanism. Cell 108(6):837–847.
doi: https://doi.org/10.1242/prelights.13888
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the biochemistry category:
Active flows drive clustering and sorting of membrane components with differential affinity to dynamic actin cytoskeleton
Teodora Piskova
Snake venom metalloproteinases are predominantly responsible for the cytotoxic effects of certain African viper venoms
Daniel Osorno Valencia
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
preLists in the biochemistry category:
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
Peer Review in Biomedical Sciences
Communication of scientific knowledge has changed dramatically in recent decades and the public perception of scientific discoveries depends on the peer review process of articles published in scientific journals. Preprints are key vehicles for the dissemination of scientific discoveries, but they are still not properly recognized by the scientific community since peer review is very limited. On the other hand, peer review is very heterogeneous and a fundamental aspect to improve it is to train young scientists on how to think critically and how to evaluate scientific knowledge in a professional way. Thus, this course aims to: i) train students on how to perform peer review of scientific manuscripts in a professional manner; ii) develop students' critical thinking; iii) contribute to the appreciation of preprints as important vehicles for the dissemination of scientific knowledge without restrictions; iv) contribute to the development of students' curricula, as their opinions will be published and indexed on the preLights platform. The evaluations will be based on qualitative analyses of the oral presentations of preprints in the field of biomedical sciences deposited in the bioRxiv server, of the critical reports written by the students, as well as of the participation of the students during the preprints discussions.
| List by | Marcus Oliveira et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
20th “Genetics Workshops in Hungary”, Szeged (25th, September)
In this annual conference, Hungarian geneticists, biochemists and biotechnologists presented their works. Link: http://group.szbk.u-szeged.hu/minikonf/archive/prg2021.pdf
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |











 (No Ratings Yet)
(No Ratings Yet)