The molecular structure of primary cilia revealed by cryo-electron tomography
Posted on: 6 April 2020
Preprint posted on 20 March 2020
Article now published in Nature Structural & Molecular Biology at http://dx.doi.org/10.1038/s41594-020-0507-4
Are you ready for your close up? Cryo-EM identifies microtubule binding proteins and actin filaments inside the mammalian primary cilium
Selected by Nicola StevensonCategories: biochemistry
Background
Cilia are ubiquitous, microtubule-based organelles that protrude from the surface of eukaryotic cells1. They come in two flavours: primary cilia, which act as antenna for various extracellular signals; and motile cilia, which beat like a propeller to move either the cell itself or to waft extracellular material such as mucus. Both types of cilia share a similar overall structure consisting of a core ring of nine microtubule doublets (the axoneme), which extends from the mother centriole and is enclosed by the ciliary membrane. Motile cilia have an additional pair of microtubule doublets in the centre (9+2 vs 9+0 arrangement).
For a cilium to function and maintain its structure, ciliary proteins such as tubulin and signalling molecules must be transported along the axoneme by specialised intraflagellar transport (IFT) machinery. Cargo molecules are assembled onto large protein complexes called IFT particles which move collectively in trains. Within the particle, sub-complex IFT-B couples to the motor protein kinesin-2 for anterograde transport to the ciliary tip. The IFT particles then rearrange and the sub-complex IFT-A couples to dynein-2 for retrograde transport back to the ciliary base.
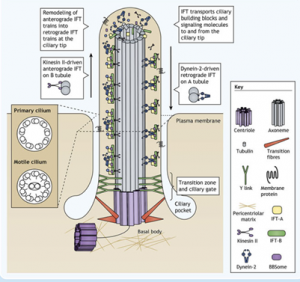
Vuolo et al Cytoplasmic dynein-2 at a glance. JCS. 2020
The basic structure of the axoneme of both motile and primary cilia from various cell types has been known for some time thanks to transmission electron microscopy (EM). However, our knowledge of the more refined details of ciliary sub-structures is almost exclusively derived from cryo-EM studies of the flagella of the algae Chlamydomonas reinhardtii2-4. These flagella display a 9+2 microtubule arrangement characteristic of eukaryotic motile cilia and flagella more broadly and thus their study has been useful. However, from the little information we do have from other organisms, we know that there is great variation in ciliary structures between species and cell types. A large number of diseases, known as ciliopathies, have also been linked to defective primary cilia function rather than motile cilia function. Thus, there is an unmet need to investigate other types of cilia in the same level of detail as the C. reinhardtii flagellum.
The sticking point for such investigations has always been technical. C. reinhardtii flagella are large compared to the cell body and fully protrude from the cell surface. There are also two per cell. Thus, they are accessible, obtainable in large numbers, easily shed and amenable to preservation and imaging in a way that other cilia are not. This study from Kiesel et al circumvents previous technical blockades and provides for the first time a detailed structural analysis of the interior of a mammalian primary cilium by cryo-electron tomography (cryo-ET).
Key findings
Firstly, using transmission EM the authors show that the 9+0 arrangement of microtubule doublets is quickly lost along the axoneme of primary cilia from MDCKII cells (canine kidney cell line), with only singlets present in the distal ¾ of the cilium. Surprisingly, both the microtubules and the axoneme in these cilia are also twisted unlike in motile cilia.
For greater structural insight, the authors develop and validate a method to isolate cilia for cryo-EM by pressing poly-L-lysine coated EM grids onto the apical membrane of MDCKII monolayers and then pulling away. This leaves the intact cilia stuck on the grid. Grids are then plunge-frozen and imaged by cryo-ET.
Using this method anterograde IFT trains measuring up to 900 nm were identified by cryo-ET on microtubule singlets. This suggests that different mechanisms of IFT regulation are in place in primary cilia than in motile cilia where anterograde and retrograde IFT occur on different tubules of each microtubule doublet to avoid collision. The structure of the IFT trains were consistent with those of Chlamydomonas.
Another key observation was that microtubule internal proteins (MIPs) are sporadically seen inside the microtubules associated with either the tubule wall or the lumen. MIP decoration was not periodic unlike in motile cilia.
The outer surface of the microtubule singlets, on the other hand, was decorated periodically with a globular density thought to be consistent with EB1 protein. Immunofluorescence confirmed EB1, normally a microtubule tip protein, is in fact bound all along the axoneme length.
Finally, the authors identify periodical helical and filamentous structures intertwined with the microtubules. These bear the structural characteristics of actin filaments and again immunofluorescence confirmed the presence of actin within the cilium.
In conclusion these results support other studies calling into question our models of axonemal structure and IFT mechanisms, and also provide novel and exciting structural insights into a potential role for EB1 and axonemal actin in reinforcing microtubules to strengthen the cilium.
Why I chose this paper
I chose this paper because it provides both a technical and intellectual leap forward for cilia biology. This information has been long awaited and it is exciting to see the authors overcome the technical challenges that have previously precluded these discoveries. It is also a great example of how structural detail can be used to inform function and will leave the field questioning what we thought we knew about the inner workings of the primary cilium and especially the mechanism of IFT.
References
- Satir et al. The primary cilium at a glance. JCS. 2010
- Jordan et al. The cryo-EM structure of intraflagellar transport trains reveals how dynein is inactivated to ensure unidirectional anterograde movement in cilia. NCB. 2018
- Nicastro, D. et al. The Molecular Architecture of Axonemes Revealed by Cryo-electron Tomography. Science. 2006
- Ma et al. Structure of the Decorated Ciliary Doublet Microtubule. Cell. t2019
doi: https://doi.org/10.1242/prelights.18131
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the biochemistry category:
Active flows drive clustering and sorting of membrane components with differential affinity to dynamic actin cytoskeleton
Teodora Piskova
Snake venom metalloproteinases are predominantly responsible for the cytotoxic effects of certain African viper venoms
Daniel Osorno Valencia
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
preLists in the biochemistry category:
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
Peer Review in Biomedical Sciences
Communication of scientific knowledge has changed dramatically in recent decades and the public perception of scientific discoveries depends on the peer review process of articles published in scientific journals. Preprints are key vehicles for the dissemination of scientific discoveries, but they are still not properly recognized by the scientific community since peer review is very limited. On the other hand, peer review is very heterogeneous and a fundamental aspect to improve it is to train young scientists on how to think critically and how to evaluate scientific knowledge in a professional way. Thus, this course aims to: i) train students on how to perform peer review of scientific manuscripts in a professional manner; ii) develop students' critical thinking; iii) contribute to the appreciation of preprints as important vehicles for the dissemination of scientific knowledge without restrictions; iv) contribute to the development of students' curricula, as their opinions will be published and indexed on the preLights platform. The evaluations will be based on qualitative analyses of the oral presentations of preprints in the field of biomedical sciences deposited in the bioRxiv server, of the critical reports written by the students, as well as of the participation of the students during the preprints discussions.
| List by | Marcus Oliveira et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
20th “Genetics Workshops in Hungary”, Szeged (25th, September)
In this annual conference, Hungarian geneticists, biochemists and biotechnologists presented their works. Link: http://group.szbk.u-szeged.hu/minikonf/archive/prg2021.pdf
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |











 (No Ratings Yet)
(No Ratings Yet)