Bariatric surgery reveals a gut-restricted TGR5 agonist that exhibits anti-diabetic effects
Posted on: 13 February 2020
Preprint posted on 11 January 2020
Article now published in Nature Chemical Biology at http://dx.doi.org/10.1038/s41589-020-0604-z
A gut check for bariatric surgery – Surgery-mediated bile acid changes improve metabolic parameters
Selected by Connor RosenCategories: biochemistry, physiology
Background:
Bariatric surgery, including sleeve gastrectomy (SG, surgical removal of ~80% of the stomach), is an effective treatment for obesity and metabolic diseases such as type 2 diabetes. The molecular basis of this improvement remains somewhat unclear. Bariatric surgery is known to alter the intestinal pool of bile acids, steroid acids produced by the liver and secreted into the gut to aid in digestion through solubilization of dietary lipids. Bile acids also act as signaling molecules, operating on sensors including the G-protein coupled receptor TGR5 and the nuclear hormone receptor FXR. While the effects of bariatric surgery on the total bile acid pool have been studied, the changes in individual bile acids, and their respective roles in mediating metabolic changes, have remained unclear. In this preprint, Chaudhari and Harris et al reveal the changes of a single bile acid, CA7S, and its effect on metabolic physiology.
Key findings:
- The bile acid CA7S is increased following bariatric surgery in mice and humans
Using ultra-high performance liquid chromatography-mass spectrometry (UPLC-MS), the authors examined individual bile acids in cecal contents of mice following SG surgery to treat diet-induced obesity. They observed an increase in only one bile acid, the sulfated cholic acid derivative CA7S. This bile acid was also elevated in the livers of mice after surgery, consistent with known biogenesis of sulfated bile acids, and in the fecal bile acid pools of human patients after SG.
- CA7S triggers GLP-1 secretion through TGR5 stimulation
Increased systemic GLP-1 levels are a known downstream effect of SG, and were indeed observed in the SG mouse model. One receptor known to be responsible for bile acid-mediated GLP-1 secretion is TGR5. The authors show that CA7S induces signaling, including calcium flux and subsequent GLP-1 secretion, in an intestinal endocrine cell line, and that this activity was dependent on TGR5 expression.
- CA7S administration has anti-diabetic effects through GLP-1
To decouple the effects of CA7S from other potential SG-induced changes, the authors administered CA7S by catheterization or by oral gavage. Both methods of administration increased circulating GLP-1 levels, and improved metabolic parameters – including circulating insulin levels in the catheterization model and improved glucose tolerance in the oral gavage model. Knockdown of the GLP-1 receptor in vivo reduced the magnitude of this effect, suggesting GLP-1 plays a major role in the in vivo effects of CA7S.
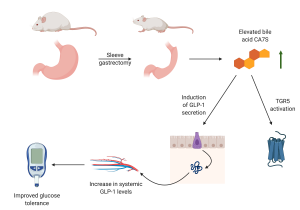
Summary of CA7S activity and role in regulating glucose tolerance following sleeve gastrectomy. Figure prepared with Biorender.
Importance:
This preprint describes the first individual metabolite that is altered by bariatric surgery and that can individually mediate improved glucose regulation. It also describes a clearly defined molecular role for the bile acid CA7S, including its identification as a TGR5 agonist. Given the profound effectiveness of bariatric surgery for obesity and other metabolic disorders, and the complexity of bile acid changes following surgery, targeted investigations such as this will be necessary to realize a pharmacological, rather than surgical, intervention that can mimic the beneficial metabolic effects of surgery.
Moving forward:
- One clear question that emerges is how CA7S is altered by surgery. Is production increased, potentially at the expense of other modifications (none of the other cholic acid derivatives change uniformly in mice and humans, so this may be complicated to identify), or is degradation decreased in the gut? Are there detectable changes in sulfotransferase gene expression following surgery?
- Bariatric surgery is known to impact the microbiota, and the microbiota also play a key role in modulating the bile acid pool through modification of bile acids into secondary bile acids. Related to the possibility of decreased degradation of CA7S, is it known to be metabolized by the microbiota? There are a number of patients with no detectable CA7S in Fig 2e – perhaps microbiome analysis may reveal a bile salt hydrolase or even a microbial species responsible for lack of detectable CA7S. It might also be predicted that patients with that gene / microbe, or who experience unusual changes in its abundance following surgery, may have distinct responses.
- The authors only examined acute effects of CA7S. It will be interesting to see how durable these effects are (one might expect circulating GLP-1 levels to fall within, say, a day after CA7S administration, but it would be interesting to know those kinetics), and what that might suggest for longer-term pharmacological targeting of TGR5 and whether CA7S or related molecules truly show the favorable toxicity profiles suggested from the acute experiments.
doi: https://doi.org/10.1242/prelights.16933
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the biochemistry category:
Notch3 is a genetic modifier of NODAL signalling for patterning asymmetry during mouse heart looping
Bhaval Parmar
14-3-3 binding regulates Tau assembly and microtubule association
Barbora Knotkova et al.
Structural basis of respiratory complexes adaptation to cold temperatures
Pamela Ornelas
Also in the physiology category:
Gestational exposure to high heat-humidity conditions impairs mouse embryonic development
Girish Kale, preLights peer support
Modular control of time and space during vertebrate axis segmentation
AND
Natural genetic variation quantitatively regulates heart rate and dimension
Girish Kale, Jennifer Ann Black
Blue appendages and temperature acclimation increase survival during acute heat stress in the upside-down jellyfish, Cassiopea xamachana
Maitri Manjunath
preLists in the biochemistry category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
Peer Review in Biomedical Sciences
Communication of scientific knowledge has changed dramatically in recent decades and the public perception of scientific discoveries depends on the peer review process of articles published in scientific journals. Preprints are key vehicles for the dissemination of scientific discoveries, but they are still not properly recognized by the scientific community since peer review is very limited. On the other hand, peer review is very heterogeneous and a fundamental aspect to improve it is to train young scientists on how to think critically and how to evaluate scientific knowledge in a professional way. Thus, this course aims to: i) train students on how to perform peer review of scientific manuscripts in a professional manner; ii) develop students' critical thinking; iii) contribute to the appreciation of preprints as important vehicles for the dissemination of scientific knowledge without restrictions; iv) contribute to the development of students' curricula, as their opinions will be published and indexed on the preLights platform. The evaluations will be based on qualitative analyses of the oral presentations of preprints in the field of biomedical sciences deposited in the bioRxiv server, of the critical reports written by the students, as well as of the participation of the students during the preprints discussions.
| List by | Marcus Oliveira et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
20th “Genetics Workshops in Hungary”, Szeged (25th, September)
In this annual conference, Hungarian geneticists, biochemists and biotechnologists presented their works. Link: http://group.szbk.u-szeged.hu/minikonf/archive/prg2021.pdf
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Also in the physiology category:
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |











 (No Ratings Yet)
(No Ratings Yet)