Strong preference for autaptic self-connectivity of neocortical PV interneurons entrains them to γ-oscillations
Posted on: 20 December 2018 , updated on: 21 December 2018
Preprint posted on 1 December 2018
Article now published in PLOS Biology at http://dx.doi.org/10.1371/journal.pbio.3000419
A team effort by the Bacci and Beato labs sheds light on the detailed organization of inhibitory autapses in neocortical interneurons.
Selected by Mahesh KarnaniCategories: biophysics, neuroscience
Summary
A team effort by the Bacci and Beato labs sheds light on the detailed organization of inhibitory autapses in neocortical interneurons.
Context
Autapses are synapses formed by a neuron onto itself, and this is something that happens most frequently in neocortical parvalbumin (PV) containing fast spiking interneurons 1–3. Not many studies have focused on autapses 4, but from the few that have it seems PV cell autapses can either synchronize spiking 1 or, after firing at high-frequency, desynchronize it through long-lasting self-inhibition 5. The role of PV cell autapses remains elusive 6.
Key findings
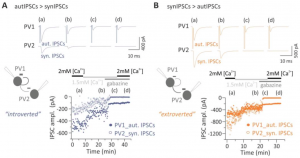
This study from two extremely competent synaptic physiology labs shows that the autapses on these cells are surprisingly strong, constituting up to 40% of the total inhibitory input. The autapses were stronger than other inhibitory synapses formed by the cells onto nearby pyramidal neurons. The authors used Bayesian quantal analysis to show the autaptic strength was higher than PV->pyramidal synapses due to bigger quantal size (i.e., bigger postsynaptic response to a released synaptic vesicle), while PV->PV synapses had similar quantal size as the autapses. The autapses had varying strength relative to heterosynaptic PV->PV synapses formed by the same presynaptic cell and this was due to different numers of presynaptic release sites. Some PV cells had stronger autaptic connections than heterosynaptic PV->PV synapses. These were termed introverted cells. Some PV cells had the opposite arrangement of synaptic strength and were termed extroverted cells. The authors went on to demonstrate that during optogenetically induced network oscillations, the transient self-inhibitory influence of PV cell autapses phases their action potential firing into the gamma-frequency range (~30Hz) rather than inhibiting themselves over a longer period. This is an important demonstration of a potential role for autapses, as previous work has shown that PV cell autapses can also elicit a prolonged asynchronous self inhibition after high frequency burst activity 5.
Why I chose this preprint
This is an important study because it uncovers a new functional organization of these interneurons into subtypes defined by connectivity: introverted and extroverted PV cells. Anatomically, PV cells have a moderate tendency to form clusters where they are packed near each other more densely 7–10. In vivo recordings of small samples of PV cells (which could have been within a cluster) have shown them to be highly coactive with each other 11. If all cortical PV cells are not simultaneously active, one possibility is that anatomical PV cell clusters form functionally coactive teams. In this case extroverted PV cells might be located on the borders or outside the clusters while introverted PV neurons would be at the core of the team. This way the introverts could keep a stronger rhythm without interfering with other nearby team members while extroverts at the border would compete against other PV teams and delineate a functional boundary. It would be interesting to see how intersomatic distance metrics from the authors’ recordings square up to this hypothesis.
What next?
Besides autapses, another key factor controlling PV neuron synchronization is their electrical coupling with each other. It would be interesting to see if electrical coupling is higher among neighbouring introverts than extroverts. As divergent excitatory input from shared presynaptic principal neurons is common among PV neurons, it would also be interesting to study whether introverts and extroverts are part of different excitatory subnetworks.
A major question for the future is whether PV cell autapses are plastic 6. This could provide the rationale for a neuron to use such a complicated mechanism to self-regulate (let’s face it, self-inhibition could conceivably be done by increased voltage gated potassium channels for example to achieve a stronger after-hyperpolarization). If the plasticity of autapses can achieve something special, such as automatically tuning the cell to an impinging rhythm, this could offer a profound explanation for these mysterious cables.
References:
- Bacci, A. & Huguenard, J. R. Enhancement of spike-timing precision by autaptic transmission in neocortical inhibitory interneurons. Neuron 49, 119–30 (2006).
- Bacci, A., Huguenard, J. R. & Prince, D. A. Functional autaptic neurotransmission in fast-spiking interneurons: a novel form of feedback inhibition in the neocortex. J. Neurosci. 23, 859–66 (2003).
- Tamás, G., Buhl, E. H. & Somogyi, P. Massive autaptic self-innervation of GABAergic neurons in cat visual cortex. J. Neurosci. 17, 6352–64 (1997).
- Ikeda, K. & Bekkers, J. M. Autapses. Curr. Biol. 16, R308 (2006).
- Manseau, F. et al. Desynchronization of Neocortical Networks by Asynchronous Release of GABA at Autaptic and Synaptic Contacts from Fast-Spiking Interneurons. PLoS Biol. 8, e1000492 (2010).
- Deleuze, C., Pazienti, A. & Bacci, A. Autaptic self-inhibition of cortical GABAergic neurons: Synaptic narcissism or useful introspection? Curr. Opin. Neurobiol. 26, 64–71 (2014).
- Wang, X. et al. Three-dimensional intact-tissue sequencing of single-cell transcriptional states. Science 361, eaat5691 (2018).
- Amitai, Y. et al. The spatial dimensions of electrically coupled networks of interneurons in the neocortex. J. Neurosci. 22, 4142–52 (2002).
- Karnani, M. M. & Jackson, J. Interneuron Cooperativity in Cortical Circuits. Neuroscientist 24, 329–341 (2018).
- Ebina, T. et al. 3D Clustering of GABAergic Neurons Enhances Inhibitory Actions on Excitatory Neurons in the Mouse Visual Cortex. Cell Rep. 9, 1896–1907 (2014).
- Hofer, S. B. et al. Differential connectivity and response dynamics of excitatory and inhibitory neurons in visual cortex. Nat. Neurosci. 14, 1045–52 (2011).
doi: https://doi.org/10.1242/prelights.6692
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the biophysics category:
Active flows drive clustering and sorting of membrane components with differential affinity to dynamic actin cytoskeleton
Teodora Piskova
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
Mitochondrial Fatty Acid Oxidation is Stimulated by Red Light Irradiation
Rickson Ribeiro, Marcus Oliveira
Also in the neuroscience category:
Electrophysiological correlates of conscious experiences during sleep: Lucid dreams, sleep paralysis, out-of-body experiences, and false awakenings
uMontreal Neuro preLighters et al.
PPARδ activation in microglia drives a transcriptional response that primes phagocytic function while countering inflammatory activation
Isabel Paine
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
preLists in the biophysics category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
66th Biophysical Society Annual Meeting, 2022
Preprints presented at the 66th BPS Annual Meeting, Feb 19 - 23, 2022 (The below list is not exhaustive and the preprints are listed in no particular order.)
| List by | Soni Mohapatra |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
Biophysical Society Meeting 2020
Some preprints presented at the Biophysical Society Meeting 2020 in San Diego, USA.
| List by | Tessa Sinnige |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Biomolecular NMR
Preprints related to the application and development of biomolecular NMR spectroscopy
| List by | Reid Alderson |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
Also in the neuroscience category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |











 (No Ratings Yet)
(No Ratings Yet)