A transient CRISPR/Cas9 expression system for genome editing in Trypanosoma brucei.
Posted on: 9 May 2020
Preprint posted on 9 May 2020
Categories: molecular biology
Background
The establishment of genome editing by CRISPR/Cas9 has greatly increased the ease across fields of research, with which mutant organisms can be generated. For Trypanosoma brucei parasites CRISPR/Cas9 has greatly facilitated the genetic modification (knock outs and tagging) of parasites, as it makes possible the deletion of two copies of non-essential genes in a single round of transfection. The most widely used system for T. brucei, uses cell lines which express Cas9 and T7RNA polymerase (T7RNAP). However, this limits the method to the use of such lines. In their work, Shaw et al introduce a novel system allowing genome editing of any T. brucei cell line (1).
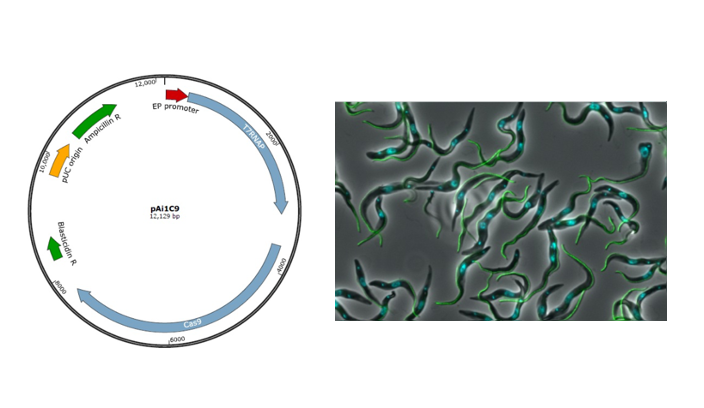
Key findings and developments
The authors generated a novel sequential transfection expression system which allows transient expression of Cas9, T7RNAP and guide RNAs, by modification of a plasmid currently used for CRISPR/Cas9-based editing in T. brucei (pTB011) (2). The new plasmid (pAi1C9) allows genome editing of any cell line, without the need for stable integration of the Cas9 and T7RNAP genes. To test the functionality of the plasmid, the authors successfully generated various mNG-tagged parasites as well as knock-out T. brucei lines of non-essential genes. The authors discuss as a limitation of this system, that it gives rise to fewer clones than cell lines stably transformed with T7RNAP and Cas9. As advantages, the authors highlight that this system can be applied to any trypanosome cell line, and circumvents possible Cas9 toxicity.
What I like about this preprint
I like that it constitutes a significant and valuable improvement for the generation of mutant Trypanosoma parasites. In some fields, the introduction of novel methods takes a long time, however this improvement comes relatively soon after the introduction of CRISPR/Cas9 altogether to the toolbox relevant for this parasite. It will likely facilitate the range of modification possible, throughput, and ultimately study of important genes in Trypanosoma brucei.
Open questions
- Why does pAi1C9 result in fewer clones than using cell lines stably transformed with T7RNAP and Cas9 genes, and how can you overcome this limitation in your current system?
- Beyond the number of clones obtained, do you think characterization of parasite mutants generated using transient vs. stable transfection might show different results for some targets?
- As future directions, what tools do you envisage would be useful for the genetic modification and phenotypic characterization of brucei mutants? For instance, in Plasmodium research, the use of barcoding technology (3) was revolutionary. Can your strategy be coupled to this type of high throughput phenotypic characterization?
- You mention that your procedure could be adapted for targeting RNAs or epigenetic mutations. This would be highly relevant to the field of brucei. Do you plan to use it in this context soon?
- Is your system translatable to other Trypanosoma species, such as T. congolense, T. vivax, T. cruzi? If not, and it would be a useful advance, do you envisage adapting it to study these species too?
References
- Shaw S, Knüsel S, Hoenner S, Roditi I, A transient CRISPR/Cas9 expression system for genome editing in Trypanosoma brucei, BMC research notes,
- Beneke T, Madden R, Makin L, Valli J, Sunter J, Gluenz E, A CRISPR Cas9 high-throughput genome editing toolkit for kinetoplastids. R Soc Open Sci, 2017
- Gomes AR, Bushell E, Schwach F, Girling G, Anar B, Quail MA, Herd C, Pfander C, Modrzynska K, Rayner JC, Billker O, A genome-scale vector resource enables high-throughput reverse genetic screening in a malaria parasite. Cell Host and Microbe, 2015
Acknoweldgements
I am very grateful to Isabel Roditi and Sebastian Shaw for their input and engaging in helpful discussion.
doi: https://doi.org/10.1242/prelights.20342
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the molecular biology category:
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
Loss of MGST1 during fibroblast differentiation enhances vulnerability to oxidative stress in human heart failure
Jeny Jose
preLists in the molecular biology category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |











 (No Ratings Yet)
(No Ratings Yet)