Preserved efficacy and reduced toxicity with intermittent linezolid dosing in combination with bedaquiline and pretomanid in a murine TB model
Posted on: 1 July 2020
Preprint posted on 12 June 2020
Article now published in Antimicrobial Agents and Chemotherapy at http://dx.doi.org/10.1128/aac.01178-20
Breathe easy: optimising linezolid dosing in tuberculosis therapy using a murine model
Selected by Zhang-He GohCategories: microbiology, pharmacology and toxicology
Background of preprint
Tuberculosis is an infectious lung disease that affects millions of patients worldwide. Unfortunately, the build-up of multidrug resistance against existing antitubercular regimens has threatened the effectiveness of existing antibiotics in recent years [1-3], leading to the rise of multidrug-resistant (MDR) and extensively drug-resistant (XDR) manifestations of tuberculosis. Given the lack of safe and effective antibiotics for tuberculosis, new antibiotics are urgently needed to curb the spread and enhance the treatment of tuberculosis today.
In a recent Nix-TB trial, a short-course 26-week oral regimen that consisted of bedaquiline (B), pretomanid (Pa), linezolid (L) was found to have favourable long-term outcomes in most patients with MDR/XDR-tuberculosis [4]. Unfortunately, a significant number of patients experienced peripheral neuropathy and myelosuppression, toxicities associated with linezolid.
Despite the need for a better understanding of linezolid’s toxicity in order to improve its clinical use, research on linezolid’s clinical role in tuberculosis therapy is complicated by its narrow therapeutic window and the long treatment durations associated with antitubercular regimens. To resolve this scientific challenge, Bigelow et al. utilise a murine model in their optimisation of linezolid’s role in antitubercular therapy.
Key findings of preprint
Previously, Bigelow et al. found that the bactericidal effect of linezolid becomes less time-dependent when paired with clinically relevant doses of pretomanid; the ratio of the area under the curve to the minimum inhibitory concentration becomes important as well [5]. This led the authors to formulate two key hypotheses in this preprint (Fig. 1):
- Within the context of the BPaL regimen, and for the same total weekly dose of linezolid, thrice-weekly administration would preserve efficacy and reduce haematological toxicity compared to daily administration.
- “front-loaded” regimens with daily linezolid dosing at doses equivalent to 1200 mg daily in humans for 1-2 months before switching to thrice-weekly administration of the same dose would maximise efficacy and minimise toxicity.
Bigelow et al. used two murine models for their work in this preprint: C3HeB/FeJ mice, which are prone to development of necrotising granulomatous lung lesions that recapitulate active tuberculosis pathology in humans; and a high-dose aerosol infection model in BALB/c mice, in which the BPaL regimen was discovered.
Using both agar proportion and broth microdilution methods, the authors first determined the minimum inhibitory concentration (MIC) of linezolid against H37Rv and HN878 in vitro. They then tested their dosing strategy in C3HeB/FeJ mice infected with the HN878 and H37Rv strains respectively. Interestingly, the authors found that linezolid appeared to have an antagonistic effect when it was added to the BPa regimen applied to HN878. In contrast, a dose-dependent increase in bactericidal activity was observed in the C3HeB/FeJ mouse model infected with H37Rv. The authors attributed the observed differences between the two experiments—particularly the unexpected result observed in C3HeB/FeJ mice—to differences between the Mycobacterium tuberculosis strains or to differences in mouse species.
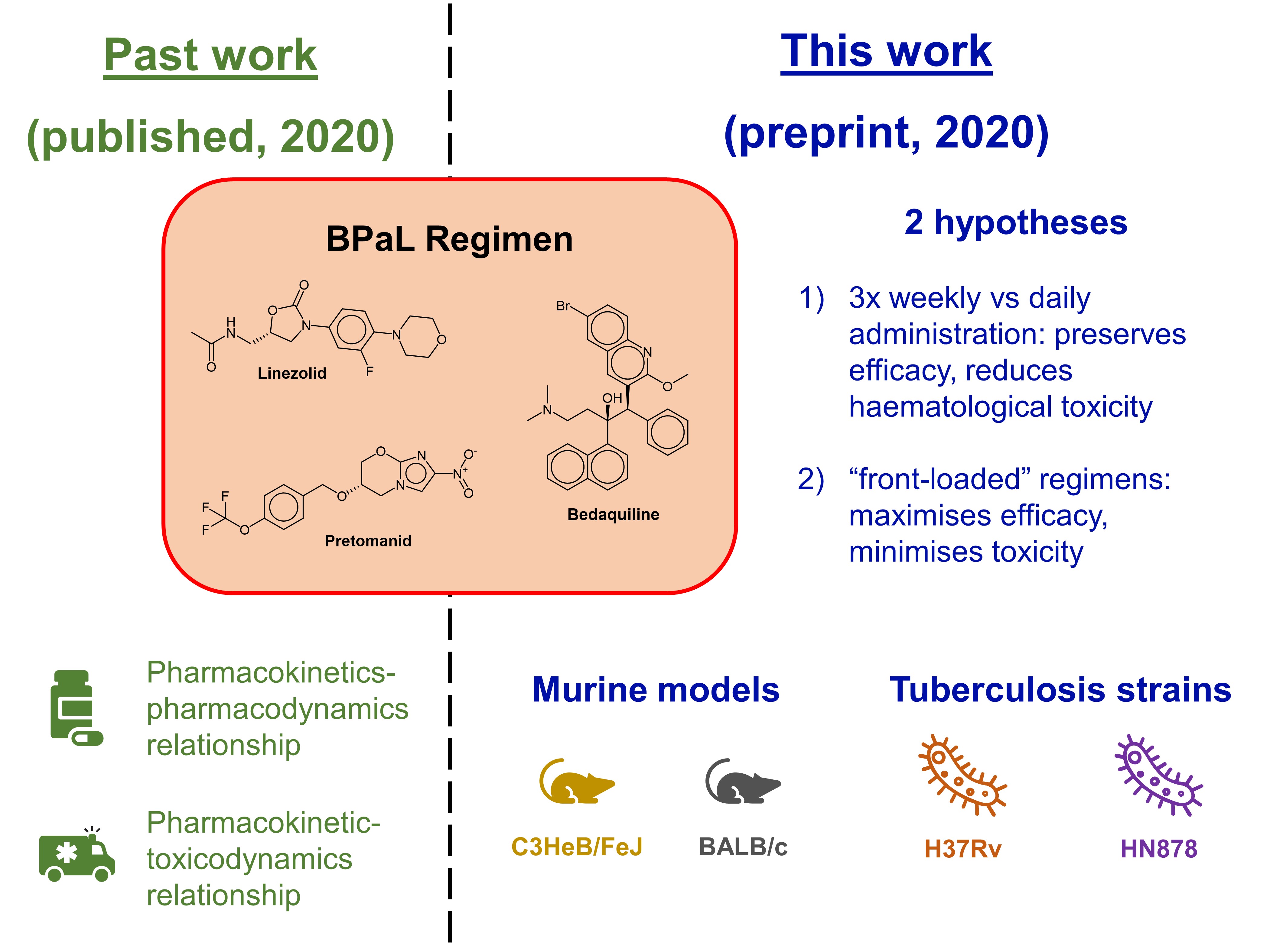
Figure 1. Relationship between the authors’ past work and this preprint.
To confirm their results observed in C3HeB/FeJ mouse model, Bigelow et al. tested both the HN878 and the H37Rv M. tuberculosis strains in BALB/c mice. Their results were consistent with those observed in the C3HeB/FeJ mouse model: linezolid again antagonised BPa in a dose-dependent fashion.
In view of the contrasting results obtained in C3HeB/FeJ mice between HN878 and H37Rv, Bigelow et al. subsequently decided to use the BALB/c mouse model to evaluate linezolid dosing strategies and bacterial strain-dependent antagonism of linezolid. In BALB/c mice infected with H37Rv, the BPaL regimen significantly outperformed the BPa regimen: linezolid both exhibited a synergistic effect with the BPa regimen, as well as a dose-dependent increase in bactericidal activity. Their observations led the authors to conclude that linezolid dosed at 1200 mg three times a week was as efficacious as linezolid dosed at 600 mg for 5 days. This result confirmed the authors’ first hypothesis.
The authors also made two observations that supported their second hypothesis: that “front-loaded” regimens with higher initial doses followed by lower subsequent doses could make regimens safer and more efficacious. To measure the safety of their linezolid dosing strategy, Bigelow et al. focussed on linezolid’s manifestation of haematological toxicity, and found that decreasing the dosing frequency of linezolid was associated with lower haematological toxicity. Bigelow et al. also found that regimens with a high dose for the first two months tended to be the most efficacious, as measured both by viable bacterial counts at the end of two months as well as the tendency of the infected BALB/c mice to relapse after 3 months of treatment.
Next, Bigelow et al. evaluated the interactions between drug components in the BPaL regimen for both the HN878 and the H37Rv strains in BALB/c mice. In mice infected with H37Rv, monotherapy with bedaquiline was more bactericidal than pretomanid, and both pretomanid and linezolid had dose-dependent effects. In mice infected with HN878, the activity of bedaquiline alone was even greater than those infected with the H37Rv strain.
Finally, the authors investigated the effect of the individual components of the BPaL regimen on the selection of drug-resistant mutants. In addition to conducting combinatorial experiments to show resistance, Bigelow et al. also investigated whether the antagonism between linezolid and bedaquiline was also found in the HN878 strain used in their present work. Using turbidity and Alamar blue checkerboard assays, the authors did not identify any such antagonism between the H37Rv and HN878 strains used in their study.
What I like about this preprint
In addition to having a personal reason for selecting a preprint on tuberculosis (I worked on tuberculosis for three years), I chose this preprint by Bigelow et al. as a pertinent example of how in vivo experiments can inform clinical decisions, and vice versa. Because tuberculosis is an especially intractable disease, antibiotics must be taken for an extended duration. Unfortunately, these antibiotics also tend to have significant amounts of toxicity, so the clinical decision to use a drug often boils down to a challenging balance between its toxicity and efficacy.
This study addresses both issues in a comprehensive and methodical manner: it is a systematic investigation of the clinical utility of the BPaL regimen. I particularly liked how the authors linked the implications of their study to the current ZeNix trial, which is testing the authors’ hypothesis underlying the efficacy and safety of the “front-loaded” regimen.
The authors also investigated the issue of antibiotic resistance in their study. I find this consideration thoughtful. Antimicrobial resistance is a global concern that manifests in many bugs other than tuberculosis: the methicillin-resistant Staphylococcus aureus (MRSA), the ESKAPE pathogens, sexually-transmitted infections, and even brain-eating amoeba. The intractability of this problem has even driven researchers to develop therapies with alternative mechanisms of action. Hence, addressing the possibility and potential implications of antibiotic resistance is critical to evaluating the clinical utility of antibiotic regimens.
Future work
In their preprint, Bigelow et al. discuss some areas for further investigation: the observed differences between the M. tuberculosis strains and clinical isolates, as well as the apparent antagonism between linezolid and the BPa regimen. They also highlight limitations of their study, such as the inability of the BALB/c mouse model to recapitulate pathological features of tuberculosis in humans, which future work will also involve. Given the prevalence and high clinical burden of tuberculosis, the resolution of these outstanding questions will help inform decisions in the clinical management of tuberculosis in the years to come.
References
[1] Ventola CL, The Antibiotic Resistance Crisis: Part 1: Causes and Threats, Pharmacy and Therapeutics 40(4) (2015) 277-283.
[2] Wellington S, Nag PP, Michalska K, Johnston SE, Jedrzejczak RP, Kaushik VK, Clatworthy AE, Siddiqi N, McCarren P, Bajrami B, Maltseva NI, Combs S, Fisher SL, Joachimiak A, Schreiber SL, Hung DT, A small-molecule allosteric inhibitor of Mycobacterium tuberculosis tryptophan synthase, Nat Chem Biol 13(9) (2017) 943-950.
[3] Global tuberculosis report 2016. World Health Organization (2016)
[4] Conradie F, Diacon AH, Ngubane N, Howell P, Everitt D, Crook AM, Mendel CM, Egizi E, Moreira J, Timm J, McHugh TD, Wills GH, Bateson A, Hunt R, Van Niekerk C, Li M, Olugbosi M, Spigelman M, Treatment of Highly Drug-Resistant Pulmonary Tuberculosis, New England Journal of Medicine 382(10) (2020) 893-902.
[5] Bigelow KM, Deitchman AN, Li S-Y, Barnes-Boyle K, Tyagi S, Soni H, Dooley KE, Savic RM, Nuermberger EL, Pharmacodynamic Correlates of Linezolid Activity and Toxicity in Murine Models of Tuberculosis, The Journal of Infectious Diseases (2020).
doi: https://doi.org/10.1242/prelights.22527
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the microbiology category:
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Microbial Feast or Famine: dietary carbohydrate composition and gut microbiota metabolic function
Jasmine Talevi
Citrobacter rodentium infection activates colonic lamina propria group 2 innate lymphoid cells
André Luiz Amorim Costa, Marcus Oliveira
Also in the pharmacology and toxicology category:
Snake venom metalloproteinases are predominantly responsible for the cytotoxic effects of certain African viper venoms
Daniel Osorno Valencia
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
In vitro pharmacokinetics and pharmacodynamics of the diarylquinoline TBAJ-587 and its metabolites against Mycobacterium tuberculosis
Zhang-He Goh
preLists in the microbiology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |
Also in the pharmacology and toxicology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
COVID-19 / SARS-CoV-2 preprints
List of important preprints dealing with the ongoing coronavirus outbreak. See http://covidpreprints.com for additional resources and timeline, and https://connect.biorxiv.org/relate/content/181 for full list of bioRxiv and medRxiv preprints on this topic
| List by | Dey Lab, Zhang-He Goh |
1
Drug use in special populations
Any drugs that are being used in special populations: Patients with liver and kidney failure, in paediatrics, in geriatrics, and in pregnant or lactating patients. Includes the discovery of factors that could potentially affect drug use in these special populations.
| List by | Zhang-He Goh |
Toxicology of toxicants, existing therapeutics, and investigational drugs
Preprints that describe the toxicology of environmental pollutants and existing and upcoming drugs. Includes both toxicokinetics and toxicodynamics, as well as technological improvements that will help in the characterisation of this field.
| List by | Zhang-He Goh |
Anticancer agents: Discovery and clinical use
Preprints that describe the discovery of anticancer agents and their clinical use. Includes both small molecules and macromolecules like biologics.
| List by | Zhang-He Goh |
Advances in Drug Delivery
Advances in formulation technology or targeted delivery methods that describe or develop the distribution of small molecules or large macromolecules to specific parts of the body.
| List by | Zhang-He Goh |











 (No Ratings Yet)
(No Ratings Yet)