Quantification of cell penetrating peptide mediated delivery of proteins in plant leaves
Posted on: 6 June 2022 , updated on: 15 June 2022
Preprint posted on 4 May 2022
Categories: bioengineering, molecular biology, plant biology
Background:
The delivery of molecules into plant cells is so far mainly based on genetic transformation by introduction of DNA into the genome. But not only is this a time-consuming, tedious process, the use of transgenic plants is also highly debated and tightly regulated by laws. Direct delivery of proteins presents a tool for DNA-free genome editing, however, this direct delivery is challenging in plant cells because of the presence of a cell wall, which serves as barrier for pathogens and macromolecules like DNA, RNA and proteins. To date, mostly small interfering RNAs are delivered into the cells via microcarriers like carbon dots and gold nanoparticles (Vega-Vásquez et al., 2020), while less tools are available for the delivery of peptides. Biolistic delivery of peptides to cells via nanoparticles requires protein dehydration which is accompanied by the potential loss of their function. A great limitation of exploring new delivery tools is the lack of a control system for the successful delivery of peptides into plant cells and its quantitative validation. Most quantification tools rely on the use of fluorescent markers, either by delivering a macromolecule encoding for a fluorescent protein or vice versa, silencing the expression of a fluorescent protein by siRNA (Watanabe et al., 2021). However, plant tissues are heterogeneous, light scattering and highly autofluorescent, making it difficult to distinguish signal from background noise. Furthermore, the vacuole in plant cells is very spacious, while the other cell organelles are pushed towards the periphery of the cells. This makes it challenging to distinguish between signal from successful delivery into the cytosol and signal from the cell wall directly adjacent, and to distinguish successful delivery from cargo accumulated in the cell wall. Therefore, the traditional approach of relying on confocal microscopy to quantify the presence of a fluorescent marker in the cell does not provide consistent and quantifiable results.
Key findings:
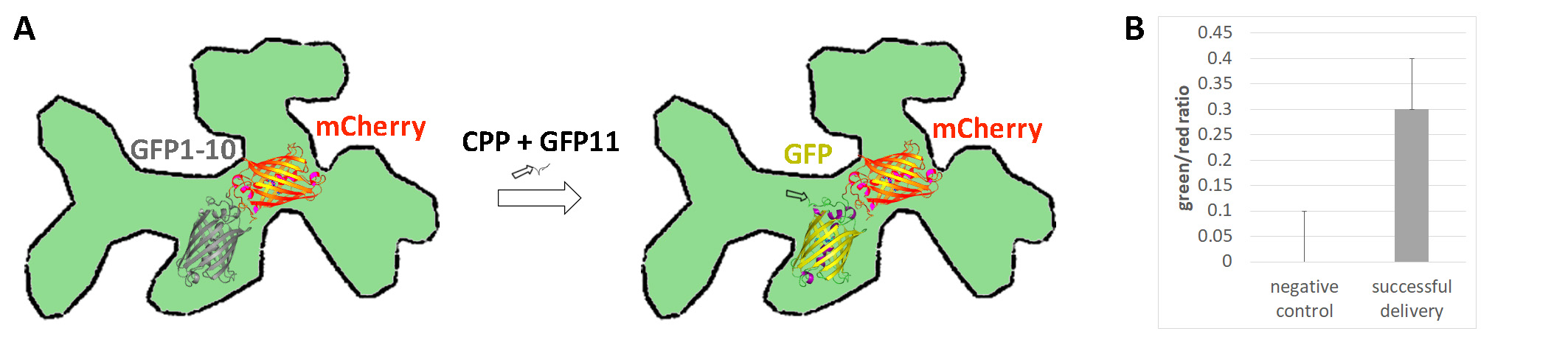
Wang et al. here introduce a feasible method for the quantification of successful delivery of peptides and bigger proteins to plant cells, by utilizing a fluorescence complementation-based method (Wang et al., 2022). In their system, Delivered Complementation in planta (DCIP), GFP is split between a larger non-fluorescent fragment (GFP1-10) and a smaller peptide strand of 16 amino acids (GFP11). Fluorescence is only reconstituted if both fragments are present in the same cellular compartment. This requires the peptide shuttled together with GFP11 to stay intact, that no sequestration to any other than the target organelle occurs and that the cargo is not trapped in the apoplast. To test the system, the authors used GFP1-10 fused to mCherry with an N-terminal SV40 nuclear localisation signal and transiently expressed the construct in tobacco leaves (Figure 1). 3 days after the infiltration, the leaves were additionally infiltrated with an aqueous solution containing cargo that contains the GFP11 tag. After confocal imaging 4-5 h later and analysis with Cell Profiler for nuclear GFP and mCherry fluorescence, they quantified the green/red ratio in mCherry expressing nuclei. Cells transformed with the GFP-mCherry construct and successfully delivered cargo show red fluorescence of mCherry in the nucleus and green fluorescence of the reconstituted GFP.
For a proof of concept, Wang et al. used different cell penetrating peptides (CPPs) as carriers for the cargo. CPPs are small cationic or amphipathic peptides that consist of 5-30 amino acids and that enable cytosolic delivery when conjugated to cargoes. They tested different CCPs as carriers, such as nona-arginine (R9), in various concentrations, and discovered that concentrations above 50 μM were required for successful delivery and trespassing the background fluorescence.
They found that delivery in plants was not as efficient as in mammalian cells, but >40 % positive delivery could be achieved when leaves were infiltrated with 100 μM of R9-GFP11 or more, with a maximum positive delivery of 75 % at 300 μM. Then they tested different CPPs, whose ability for delivery into plant cells was previously proved by other methods. Two of them were successfully delivered, the third one only showed a successful delivery in rare instances, indicating that conjugation to different cargoes can alter the uptake rate. Temperature-treatment experiments and co-infiltration with endocytosis inhibitors showed that the CPP penetration was not dependent on endocytosis, and suggest that the cargo enters cells through direct membrane permeation. Analysing the delivery over a time course showed that the delivery could be detected 4 h after infiltration with the cargo, but was undetectable after 24 h. Together with immuno blotting, this indicates that the peptide is unstable in the leaf tissues, potentially affected by apoplastic proteases and intracellular degradation.
To show that DCIP also enables the detection of bigger recombinant proteins, the authors tagged mCherry N-terminally with GFP11 and C-terminally with a CPP and used it as cargo, with a final weight of 34.5 kDa. They detected GFP complementation also with this cargo, however, the delivery was less efficient than the one of smaller peptides. To test if the delivered proteins are able to mediate protein-protein interaction or mislocalisation of proteins, the authors delivered an actin binding peptide, tagged with GFP11 and a CPP into cytosolic DCIP (cytoDCIP) expressing tobacco leaves. They detected GFP fluorescence along the actin filaments, indicating that the GFP11 fused to the actin binding peptide was able to tether the cytoDCIP to actin.
Importance and future directions:
The described quantification method for the delivery of proteins to plant cells can be used to rapidly screen the effectivity of delivery peptides, and also account for the durability of the uptake and stability of the delivered peptides in the tissue. A similar system could be used for other delivery systems that transport RNA or DNA.
Direct delivery of macromolecules to cells enables DNA-free genome editing and DNA-free interference into physiological processes, avoiding tedious plant transformation and legal regulations of genetically modified organisms. Given that mCherry was able to penetrate the cells fused to CPPs, the delivery of Cas9 for DNA-free genome editing might ultimately become possible. Direct delivery of macromolecules might play an important role in future agriculture: it can drive the development of more precise pesticides forward, because bioactive molecules can be directly delivered into plant cells and thereby their effectivity is increased, while applied concentrations can be decreased. Furthermore, controlling the uptake and turnover rate of the active compound enables a time-controlled release to the target site (Vega-Vásquez et al., 2020).
References:
Vega-Vásquez, P., Mosier, N. S., & Irudayaraj, J. (2020). Nanoscale Drug Delivery Systems: From Medicine to Agriculture. Frontiers in Bioengineering and Biotechnology, 8, 1–16. https://doi.org/10.3389/fbioe.2020.00079
Wang, J. W., Goh, N., Lien, E., González-grandío, E., & Landry, M. P. (2022). Quantification of cell penetrating peptide mediated delivery of proteins in plant leaves. BioRxiv, 1–33. https://doi.org/https://doi.org/10.1101/2022.05.03.490515
Watanabe, K., Odahara, M., Miyamoto, T., & Numata, K. (2021). Fusion Peptide-Based Biomacromolecule Delivery System for Plant Cells. ACS Biomaterials Science & Engineering, 7(6), 2246–2254. https://doi.org/10.1021/acsbiomaterials.1c00227
doi: https://doi.org/10.1242/prelights.32247
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the bioengineering category:
A Novel Chimeric Antigen Receptor (CAR) - Strategy to Target EGFRVIII-Mutated Glioblastoma Cells via Macrophages
Dina Kabbara
Human pluripotent stem cell-derived macrophages modify development of human kidney organoids
Theodora Stougiannou
Matrix viscoelasticity regulates dendritic cell migration and immune priming
Roberto Amadio
Also in the molecular biology category:
A drought stress-induced MYB transcription factor regulates pavement cell shape in leaves of European aspen (Populus tremula)
Jeny Jose
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
Also in the plant biology category:
A drought stress-induced MYB transcription factor regulates pavement cell shape in leaves of European aspen (Populus tremula)
Jeny Jose
Actin Counters Geometry to Guide Plant Cell Division
Jeny Jose
The nucleus follows an internal cellular scale during polarized root hair cell development
Jeny Jose
preLists in the bioengineering category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Advances in microscopy
This preList highlights exciting unpublished preprint articles describing advances in microscopy with a focus on light-sheet microscopy.
| List by | Stephan Daetwyler |
Also in the molecular biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Also in the plant biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |











 (No Ratings Yet)
(No Ratings Yet)