Hypoxia blunts angiogenic signaling and upregulates the antioxidant system in elephant seal endothelial cells
Posted on: 13 September 2023
Preprint posted on 3 July 2023
Diving into Molecular Defenses: How elephant seals protect themselves from oxygen poisoning during deep dives
Selected by Sarah Young-VeenstraCategories: ecology, molecular biology, pathology, physiology, zoology
Background
Elephant seals (Mirounga angusitrostris) routinely dive to hunt active prey for durations up to an hour. To make such intense activity possible without access to oxygen, seals undergo specialized physiological changes when diving, such as bradycardia and vasoconstriction. Despite these abilities, the extreme hypoxia that seals experience during their dives has the potential to take a significant pathological toll.
Most mammals resort to reversing mitochondrial transport under extreme hypoxic conditions as a means to fuel their body’s activity in the absence of oxygen. This reversal produces succinate, which functions as an alternative energetic substrate. However, this succinate accumulation becomes dangerous upon return to normoxia, when mitochondrial transport returns to normal and oxidizes the succinate, resulting in the production of dangerous reactive oxygen species (ROS). This ROS production should cause oxidative damage, but no such damage is seen in seals, suggesting they must have some defense against hypoxia-induced pathologies.
Glutathione (GSH), a major antioxidant in animals, may be at the center of seals’ defense against hypoxia-induced oxidative damage. Indeed, marine mammals possess high GSH levels both in tissues and in circulation, and diving mammals display positive selection for, and duplications of, genes along the GSH metabolic pathway. However, whether the dynamics of GSH expression are hypoxia dependent (i.e., whether GSH plays any role during submersion and dive recovery) is unknown. Molecular changes during active dives have not previously been assessed due to the infeasibility of taking biological samples from seals mid-dive. Allen and her team from the University of California Berkely developed a novel primary cell culture system, wherein they isolated arterial endothelial cells from elephant seal placentas, which allowed them to study the cellular response to hypoxia. The goal of their study was to investigate the real-time molecular changes during an elephant seal’s dive.
Key Findings
The research team dove into measuring multiple biological responses to hypoxia in arterial endothelial cells of the elephant seal. Furthermore, they conducted the same measurements on human arterial endothelial cells in order to gauge which aspects of the hypoxia response are specialized to the diving mammal. They found that elephant seals decrease their inflammatory signalling capacity, inhibit the typical mammalian hypoxia signalling pathway to prevent angiogenesis and reliance on glycolysis, accumulate glutathione (GSH) and increase expression of GSH metabolic genes, and suggest that promotion of GSH metabolism effectively combats oxidative damage.
Elephant seals limit inflammation to maintain critical blood supply
Hypoxia induced an immediate anti-inflammatory response in both elephant seal and human cells by downregulating the Tumor Necrosis Factor (TNF) signalling pathway, which promotes cell proliferation. However, the overall anti-inflammatory effect was more pronounced in seal cells, which also downregulated additional cell-proliferation signalling pathways (Transforming Growth Factor [TGF-β] and Nuclear Factor [NF-kB]) (Figure 1). Decreasing the body’s inflammatory signaling capacity under hypoxic conditions likely limits the vasodilation potential during dives, thereby reducing blood flow to peripheral tissues and conserving blood to oxygenate essential organs such as the brain and heart.
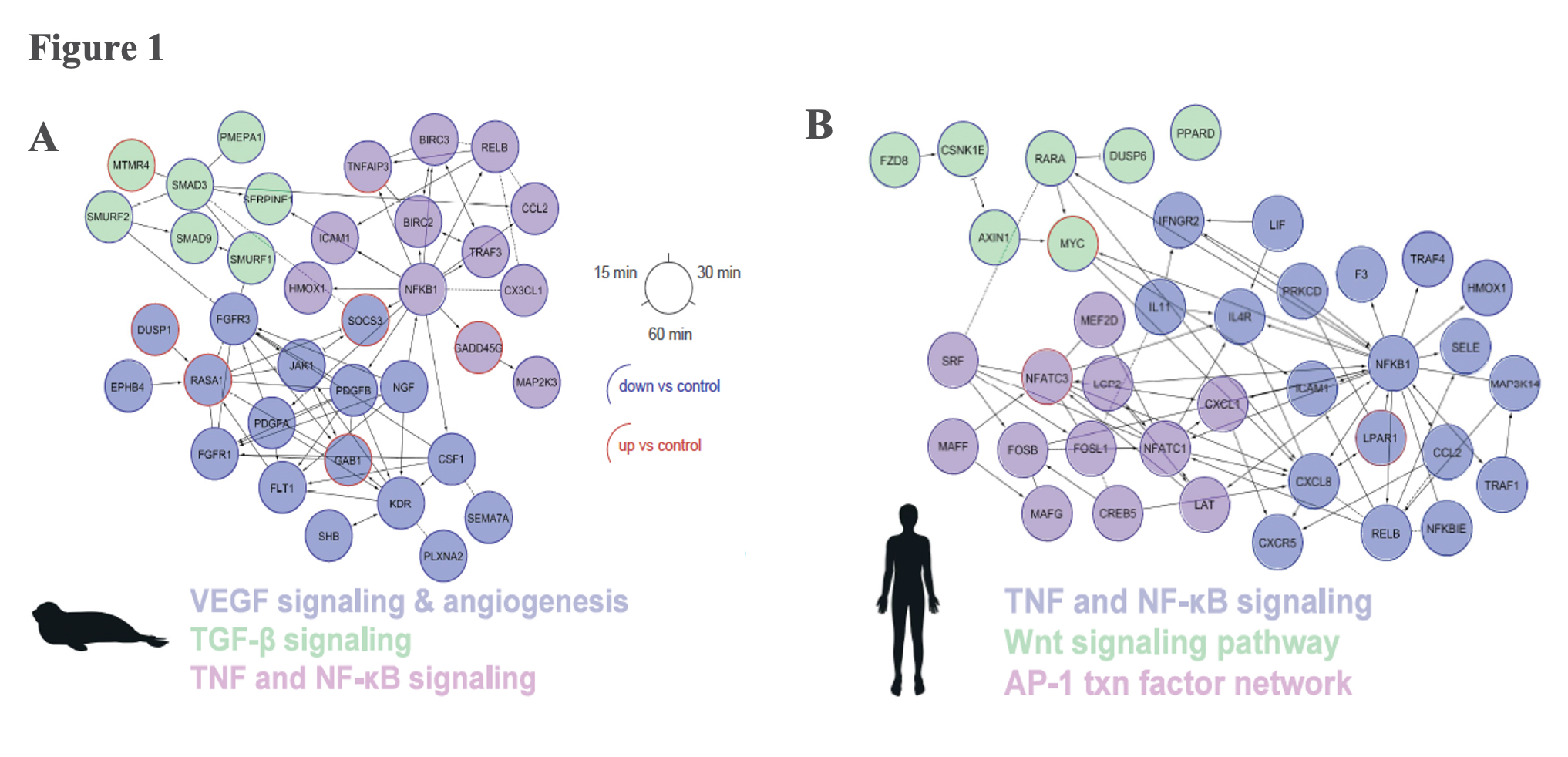
Elephant seals dissociate from the typical mammalian hypoxia response
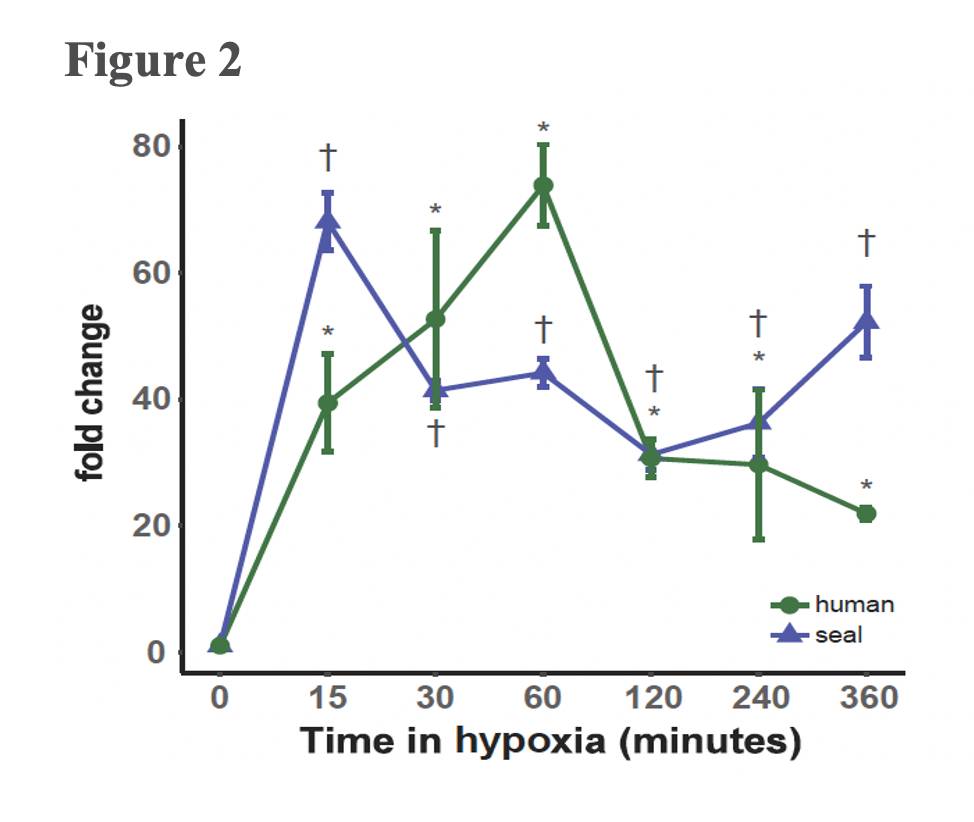
A typical mammalian response to hypoxia, as evidenced here in the human cells, sees increased angiogenesis and an increased reliance on glycolysis for anaerobic energy provision (Figure 3B). These whole-organism level modulations are promoted on a molecular level through the Hypoxia Inducible Factor (HIF)-1 regulatory pathway. The protein coding gene HIF-1α, commonly referred to as the “master regulator” of the hypoxia response, is stabilized in response to hypoxia exposure, which sets off a signalling cascade that ultimately induces biological changes that maintain oxygen homeostasis, namely angiogenesis and a metabolic shift to glycolytic pathways. Interestingly, elephant seal cells stabilized HIF-1α more rapidly than human cells (Figure 2), but seals neither promoted angiogenesis nor shifted to glycolytic metabolism (Figure 3A). The lack of HIF-1 downstream effects suggests that HIF-1α stabilization is decoupled from vascular homeostasis in elephant seals, thereby inhibiting the HIF-1 pathway from promoting biological shifts that may render the vascular system vulnerable to oxidative damage.
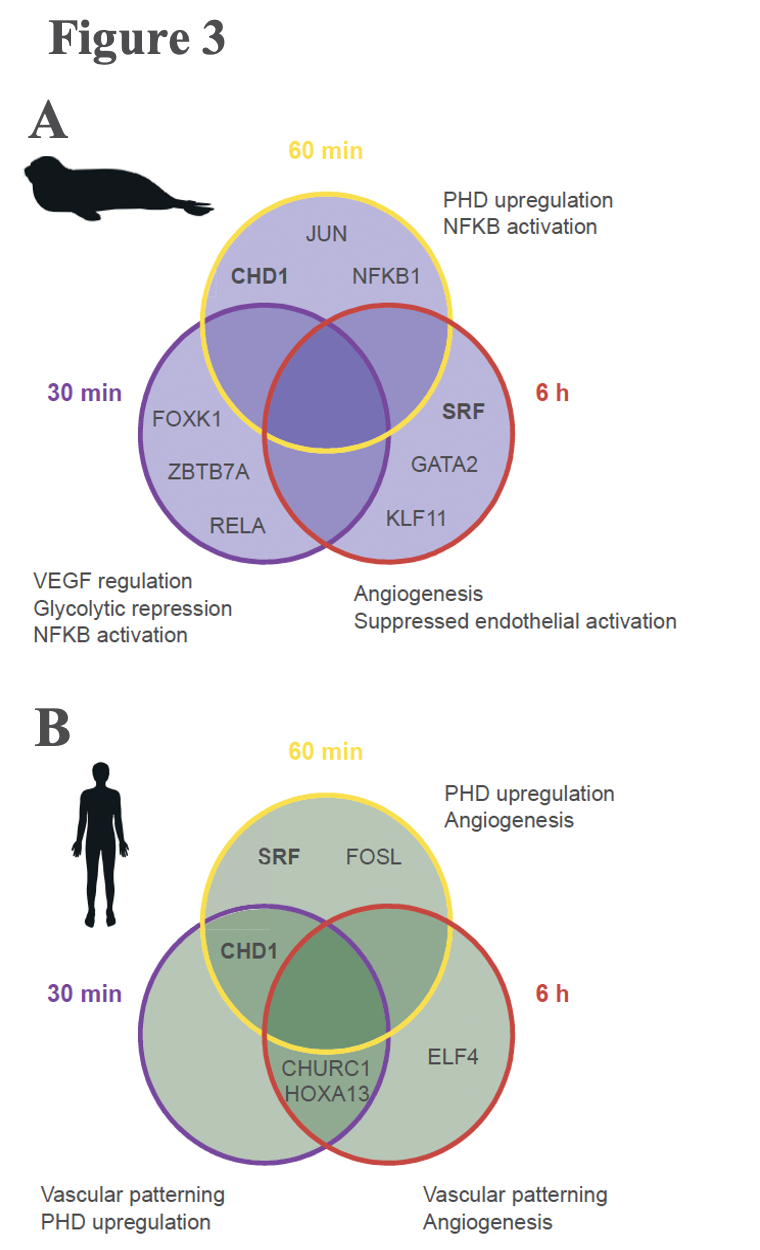
Elephant seals may anaerobically metabolize polyamines to generate energy
Succinate levels did not increase in human cells exposed to hypoxia but increased 40% in elephant seal cells after 6 hours of hypoxia exposure (Figure 4). This difference in succinate levels between seals and humans experiencing hypoxia is consistent with the human cells, but not the seal cells, needing to rely on glycolysis during hypoxia, as seals may use succinate as their energetic substrate instead. Interestingly, 40% is a relatively mild succinate accumulation, and suggests that the source of the succinate is not reversal of mitochondrial transport. The authors pose that a more likely cause of the elephant seal’s succinate accumulation is polyamine processing, and that this pathway may be advantageous. Indeed, under hypoxic conditions, elephant seals upregulated expression of several genes along the GSH metabolic pathway, some of which also play a role in polyamine synthesis. Polyamines regulate mitochondrial respiration by modulating the pyruvate dehydrogenase complex. Additionally, the polyamine putrescine can be further converted into succinate, which may go on to fuel oxidative phosphorylation as well as competitively inhibit the enzymes that hydrolyze HIF-1α, potentially accounting for the increased hypoxia sensitivity of HIF-1α in elephant seal cells relative to human cells.
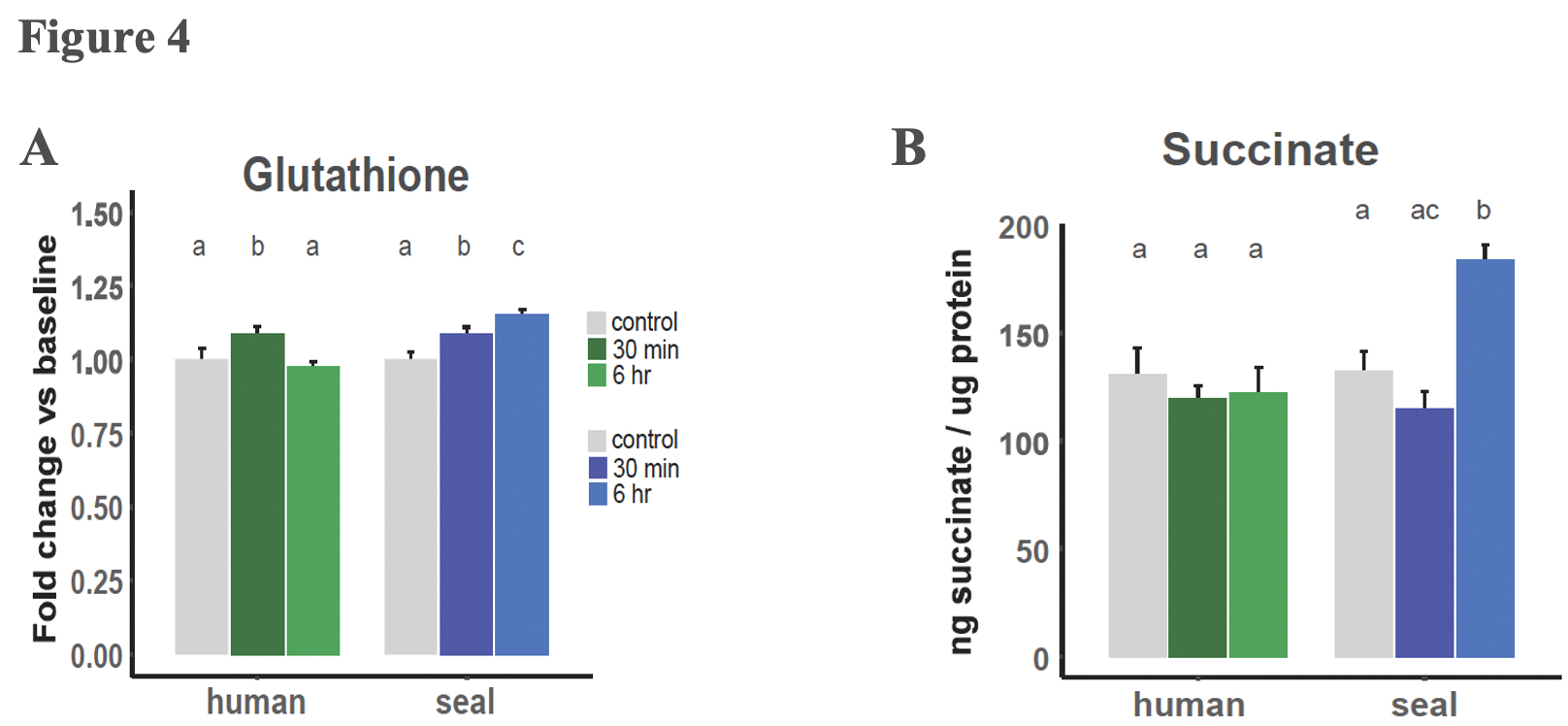
Glutathione is likely the crux of oxidative damage control in elephant seals
Increased expression of GSH metabolic genes was unique to the elephant seal cells and correlated with elevated GSH levels in seal cells relative to human cells. Such metabolic genes included glutamate-cysteine ligase, which catalyzes the rate limiting step of GSH biosynthesis, and glutathione synthetase, another a key enzyme in GSH metabolism. Furthermore, elephant seals demonstrated sustained GSH production throughout the duration of hypoxia exposure.
Conclusion
This study suggests that the elephant seal’s affinity for GSH metabolism may be at the center of their defense against oxidative pathology, not only due to the antioxidative properties of GSH, but also because metabolic genes along the GSH biosynthetic pathway have a dual function in synthesizing polyamines, which ultimately produce succinate to fuel oxidative phosphorylation under hypoxic conditions.
Why I Chose This Paper
This preprint points out an interesting physiological dichotomy in the diving physiology of seals that I had never come across before. Understanding how seals cope with routine hypoxic dives while, seemingly contradictorily, protecting themselves against superoxide production is an intriguing avenue of research and is also potentially relevant to other species as a rapidly changing climate threatens many species with increased instances of hypoxia. As the researchers report, there are several avenues where human endothelial cells employ different pathways from the elephant seal’s, presumably reflecting the lack of adaptation to routinely coping with hypoxia. Furthering knowledge as to how the seal’s specialized pathways effectively fight against pathological effects of hypoxia, and then learning which species are capable of employing similar pathways, may be important to predicting which species may be more vulnerable to impending climate change and consequent hypoxic zones.
Questions for the Authors and Future Directions
- In what way do you think reducing the vasodilation potential is affiliated with preventing oxidative pathology?
- Although the 40% succinate accumulation observed in elephant seals is more mild than you might expect from ETC reversal, do you suspect that the observed levels are generally utilized before surfacing from a dive (i.e., would even these levels have a chance to be oxidized into ROS)?
- Why do you think humans did not appear to accumulate any succinate under hypoxia? Might humans not be reversing the ETC either?
- Based on this study, an interesting continuation would investigate the synthesis and processing of polyamines, specifically putrescine, during hypoxia and reoxygenation in elephant seals to test whether this pathway does, in fact, 1) modify pyruvate dehydrogenase function to provide succinate during elephant seals’ dives and 2) competitively inhibit HIF-1α.
doi: https://doi.org/10.1242/prelights.35529
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the ecology category:
Cannibalism as a mechanism to offset reproductive costs in three-spined sticklebacks
Tina Nguyen
Trade-offs between surviving and thriving: A careful balance of physiological limitations and reproductive effort under thermal stress
Tshepiso Majelantle
The cold tolerance of an adult winter-active stonefly: How Allocapnia pygmaea (Plecoptera: Capniidae) avoids freezing in Nova Scotian winters
Stefan Friedrich Wirth
Also in the molecular biology category:
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
Loss of MGST1 during fibroblast differentiation enhances vulnerability to oxidative stress in human heart failure
Jeny Jose
Also in the pathology category:
Snake venom metalloproteinases are predominantly responsible for the cytotoxic effects of certain African viper venoms
Daniel Osorno Valencia
Schistosoma haematobium DNA and Eggs in the Urine Sample of School-Age Children (SAC) in South-West Nigeria
Hala Taha
FUS Mislocalization Rewires a Cortical Gene Network to Drive 2 Cognitive and Behavioral Impairment in ALS
Taylor Stolberg
Also in the physiology category:
Trade-offs between surviving and thriving: A careful balance of physiological limitations and reproductive effort under thermal stress
Tshepiso Majelantle
Imaging cellular activity simultaneously across all organs of a vertebrate reveals body-wide circuits
Muhammed Sinan Malik
Wide-ranging behavioral dysfunction in two mouse models of pathological human variants in the GRIK2 kainate receptor gene
Pushpinder Singh
Also in the zoology category:
Morphological variations in external genitalia do not explain the interspecific reproductive isolation in Nasonia species complex (Hymenoptera: Pteromalidae)
Stefan Friedrich Wirth
Trade-offs between surviving and thriving: A careful balance of physiological limitations and reproductive effort under thermal stress
Tshepiso Majelantle
The cold tolerance of an adult winter-active stonefly: How Allocapnia pygmaea (Plecoptera: Capniidae) avoids freezing in Nova Scotian winters
Stefan Friedrich Wirth
preLists in the ecology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
EMBO | EMBL Symposium: The organism and its environment
This preList contains preprints discussed during the 'EMBO | EMBL Symposium: The organism and its environment', organised at EMBL Heidelberg, Germany (May 2023).
| List by | Girish Kale |
Bats
A list of preprints dealing with the ecology, evolution and behavior of bats
| List by | Baheerathan Murugavel |
Also in the molecular biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Also in the pathology category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
COVID-19 / SARS-CoV-2 preprints
List of important preprints dealing with the ongoing coronavirus outbreak. See http://covidpreprints.com for additional resources and timeline, and https://connect.biorxiv.org/relate/content/181 for full list of bioRxiv and medRxiv preprints on this topic
| List by | Dey Lab, Zhang-He Goh |
1
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
Also in the physiology category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Also in the zoology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
Bats
A list of preprints dealing with the ecology, evolution and behavior of bats
| List by | Baheerathan Murugavel |











 (No Ratings Yet)
(No Ratings Yet)