Behavioural stress feedback loops in benthic invertebrates caused by pH drop-induced metabolites
Posted on: 19 May 2021 , updated on: 21 May 2021
Preprint posted on 18 April 2021
Article now published in Frontiers in Marine Science at http://dx.doi.org/10.3389/fmars.2021.773870
Ocean acidification can directly as well as indirectly affect feeding and avoidance behavior in the benthic community.
Selected by Fouzia HaiderCategories: animal behavior and cognition, biochemistry, ecology
Ocean acidification or pH drop is progressing at an alarming rate and poses a great threat to intertidal benthic organisms. A constant shift in water pH level compels these organisms to live at their physiological tolerance limit, making them interesting model species to study the effects of climate change. These organisms rely on different behavioral and physiological pathways to avoid and/or adjust to these changes, among which chemical signaling is a crucial one. However, ocean acidification can disrupt this chemical signaling in the aquatic environment and thus requires more scientific attention.
Although the effect of biotic stressors on chemical communication has been studied in the past, the effect of abiotic stressors, such as pH drop, is still not well studied. Here, Feugere et al. studied the direct and indirect effects of pH drop using a ‘stress metabolite’ approach from conspecific and heterospecific donors to understand the importance of chemical signaling and their dissemination capabilities. Upon dissemination, stress metabolites can cause an indirect stress propagation in the conspecifics and/or heterospecifics creating a positive feedback-loop. Therefore, the authors hypothesize that an acute pH drop will both directly and indirectly cause behavioral change in their sample species (small hermit crab Diogenes pugilator, green shore crab Carcinus maenas, and harbor ragworm Hediste diversicolor).
To test this hypothesis, they exposed the sample species to control pH 8.2 with control metabolite (CM), pH drop 7.6 with control metabolite (pH drop), control pH 8.2 with stress metabolite (SM), and pH 7.6 with stress metabolite (pH drop+SM). The authors also tested the effect of SM from their vertebrate predator gilt-head sea bream Sparus aurata on each sample species. The effects of aforementioned treatments were investigated on the escape mechanisms and ‘time-to-success’ (the time to find a feeding cue for crabs and to bury the head entirely in the sediment for ragworm).
Results
In the small hermit crab, Diogenes pugilator, time-to-success was significantly affected by pH drop and SM from S. aurata (Fig. 1B). Avoidance response in D. pugilator was significantly higher in response to SM from S. aurata (69%), but pH drop did not have any effect (Fig. 2). In green shore crab, Carcinus maenas, time-to-success was not affected by any of the four treatments when exposed to SM from conspecifics. However, when S. aurata was the metabolite donor, lower feeding success was noticed in response to pH drop and SM (Fig. 1). Similarly, when exposed to metabolites from conspecific donor, no effect of CM, pH drop, SM, and pH drop+SM was significant on the avoidance response of C. maenas, but exposure to S. aurata metabolites caused a significant increase in their avoidance activity in pH drop, SM and pH drop+SM treatments (Fig. 2).

Figure 1: Effect of pH drop and donor specific stress metabolite on feeding cue of hermit crab, green shore crab, and harbor ragworm. (B) Interaction of pH drop and donor specific stress metabolite on time-to-success. (C) Kaplan-Meier curves visualizing success probability under each experimental condition over time.
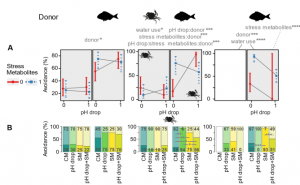
Figure 2: Effects of pH drop and donor specific stress metabolites on the avoidance behavior of hermit crab, green shore crab, and harbor ragworms. (A) Interaction of pH drop and donor specific stress metabolites (conspecific SM first window; heterospecific SM second window) on hermit crab (first column), green shore crab (middle column), and harbor ragworm (third column). (B) Split bars represent the presence (dark area) and absence (light area) of avoidance behavior in the hermit crab, green shore crab, and harbor ragworm.
In case of harbor ragworm, Hediste diversicolor, time-to-success did not depend on donor species, but pH drop and SM had a significant negative impact (Fig. 1B). The effect of conspecific metabolite donor on the burrowing success score of H. diversicolor was more pronounced than that of heterospecific metabolites. Conversely to their time-to-success, their avoidance response was much pronounced when exposed to S. aurata metabolites compared to conspecific metabolites. H. diversicolor exposed to S. aurata metabolite showed different avoidance responses in different treatment group (CM, pH drop, SM, and pH drop+SM) (Fig. 2).
What I like about this preprint
In this preprint the authors tried to unveil the role of chemical signaling in response to an abiotic stressor, pH drop. Climate change is progressively challenging the organisms living in the intertidal zone by impacting the abiotic parameters of the water, and ocean acidification or pH drop is among one of them. The fact that the authors studied the effect of pH drop on an intra- and interspecific level using stress metabolites sheds new light on the study of metabolomics and its importance on the study of eco-physiology. This study also allows us to think about how stress metabolites can propagate from an individual to a population level and create awareness among different organisms.
Future implications and questions to authors
It is very intriguing that the time-to-success and avoidance mechanisms was different depending on the metabolite donors. As the heterospecific metabolite donor caused a difference in the measured parameters, how different was the metabolite composition from S. aurata compared to the conspecific metabolite composition?
What metabolites were the dominant ones and how do these metabolites create an awareness (in which potential pathways) to the studied organisms?
The effects of pH drop on small hermit crab and ragworm are more pronounce compared to green shore crab. Is it possible that this is due to the mobility of the chosen sample species; ragworm and hermit crab having less mobility than shore crab?
doi: https://doi.org/10.1242/prelights.29062
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the animal behavior and cognition category:
Cannibalism as a mechanism to offset reproductive costs in three-spined sticklebacks
Tina Nguyen
Morphological variations in external genitalia do not explain the interspecific reproductive isolation in Nasonia species complex (Hymenoptera: Pteromalidae)
Stefan Friedrich Wirth
Trade-offs between surviving and thriving: A careful balance of physiological limitations and reproductive effort under thermal stress
Tshepiso Majelantle
Also in the biochemistry category:
Active flows drive clustering and sorting of membrane components with differential affinity to dynamic actin cytoskeleton
Teodora Piskova
Snake venom metalloproteinases are predominantly responsible for the cytotoxic effects of certain African viper venoms
Daniel Osorno Valencia
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Also in the ecology category:
Resilience to cardiac aging in Greenland shark Somniosus microcephalus
Theodora Stougiannou
Cannibalism as a mechanism to offset reproductive costs in three-spined sticklebacks
Tina Nguyen
Trade-offs between surviving and thriving: A careful balance of physiological limitations and reproductive effort under thermal stress
Tshepiso Majelantle
preLists in the animal behavior and cognition category:
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Bats
A list of preprints dealing with the ecology, evolution and behavior of bats
| List by | Baheerathan Murugavel |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Also in the biochemistry category:
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
Peer Review in Biomedical Sciences
Communication of scientific knowledge has changed dramatically in recent decades and the public perception of scientific discoveries depends on the peer review process of articles published in scientific journals. Preprints are key vehicles for the dissemination of scientific discoveries, but they are still not properly recognized by the scientific community since peer review is very limited. On the other hand, peer review is very heterogeneous and a fundamental aspect to improve it is to train young scientists on how to think critically and how to evaluate scientific knowledge in a professional way. Thus, this course aims to: i) train students on how to perform peer review of scientific manuscripts in a professional manner; ii) develop students' critical thinking; iii) contribute to the appreciation of preprints as important vehicles for the dissemination of scientific knowledge without restrictions; iv) contribute to the development of students' curricula, as their opinions will be published and indexed on the preLights platform. The evaluations will be based on qualitative analyses of the oral presentations of preprints in the field of biomedical sciences deposited in the bioRxiv server, of the critical reports written by the students, as well as of the participation of the students during the preprints discussions.
| List by | Marcus Oliveira et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
20th “Genetics Workshops in Hungary”, Szeged (25th, September)
In this annual conference, Hungarian geneticists, biochemists and biotechnologists presented their works. Link: http://group.szbk.u-szeged.hu/minikonf/archive/prg2021.pdf
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Also in the ecology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
EMBO | EMBL Symposium: The organism and its environment
This preList contains preprints discussed during the 'EMBO | EMBL Symposium: The organism and its environment', organised at EMBL Heidelberg, Germany (May 2023).
| List by | Girish Kale |
Bats
A list of preprints dealing with the ecology, evolution and behavior of bats
| List by | Baheerathan Murugavel |











 (No Ratings Yet)
(No Ratings Yet)