Enteric pathogens induce tissue tolerance and prevent neuronal loss from subsequent infections
Posted on: 23 April 2021 , updated on: 26 April 2021
Preprint posted on 11 April 2021
Article now published in Cell at http://dx.doi.org/10.1016/j.cell.2021.10.004
Keeping the gut feeling: two immune mechanisms protect enteric associated neurons from future threats
Selected by Anirban BaralCategories: immunology, microbiology, neuroscience, pathology
Background
Our intestines are constantly under attack from food-borne infections. In addition to the direct damage caused by the pathogens, the accompanying inflammatory responses cause prolonged secondary damages to the gut tissue. The gut lining (epithelium) is capable of regeneration after damages from such attacks [1]. However, the underlying enteric nervous system (ENS) has limited regeneration potential [2]. Damages to the ENS leads to impairment of gut motility, the stretching and contraction of the gastrointestinal muscles. Gut motility is essential for movement of food through the intestinal system, meaning any damage to the ENS can lead to severe digestive issues such as irritable bowel syndrome. It is therefore crucial to protect the ENS from infection by enteric pathogens and the inflammation that occurs as a result.
A previous study from the laboratory of Daniel Mucida at The Rockefeller University looked into such protective mechanisms. They found that infection by spiB, an attenuated strain of Salmonella typhimurium, has the potential to cause significant ENS damage in mice, but that the neurons protect themselves by activating gut dwelling muscularis macrophages (MMs) by adrenergic signaling. Importantly, activated MMs produce polyamine compounds that protect enteric neurons from inflammation mediated damages [3]. Activation of MMs is an innate immune response, and not specific to a particular pathogen. In the current preprint, [4] Mucida and colleagues explore the intriguing possibility that activation of innate immune responses by enteric infections might offer some degree of protection for the ENS from future attack by unrelated pathogens.
Key Findings
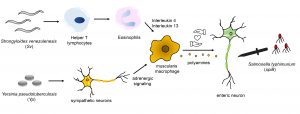
Using the previously characterized model of ENS damage in mouse intestines [3], the authors explored whether infection by food-borne pathogen Yersinia pseudotuberculosis (Yp) can protect against subsequent infection by spiB. They found that Yp infection caused relatively moderate ENS damage, but prevented further ENS damage and loss of gut motility by subsequent spiB infection. Yp infection activated MMs through adrenergic signaling, and the sustained production of polyamines by these activated MMs was crucial for ENS protection from the later spiB infection. While this observation is relevant from a disease tolerance perspective, it is not entirely unexpected that the immune responses to two bacterial pathogens would be similar and therefore offer some degree of cross-protection.
What came as a surprise was that infection by an entirely different kind of parasite, the helminth worm, Strongyloides venezulenesis (Sv) could protect against subsequent spiB-induced ENS damage. Sv infects a completely different portion of the intestine (duodenum) than the region colonized by spiB (ileum), so the protection mechanism induced by Sv appears to be operating across different domains of the intestines. Using elaborate genetic and immune perturbations, the authors teased apart the molecular basis of this protection. While Sv infection also involved polyamine production by MMs, the mechanism of MM activation was completely different from that of bacterial infections. Unlike the adrenergic route induced by Yp, Sv infection activated MMs through type-2 immune response, an innate immune pathway specifically induced by helminth infections [5]. Sv infection resulted in accumulation of helper T lymphocytes in the gut. These helper T lymphocytes in turn stimulated proliferation of another type of white blood cells, the eosinophils. Interleukin-4 and interleukin-13, two signaling proteins made by these activated eosinophils, ultimately led to increased polyamine production by the MMs, ensuring neuronal protection.
In summary, the study elegantly demonstrates two independent arms of innate immunity, activated by two entirely different pathogens converging into a common mechanism that leads to neuroprotection.
The authors have provided a broader perspective of their findings by looking into pet store mice. Unlike laboratory animals reared in near-sterile conditions, these mice are exposed to many more pathogens and therefore expected to have a more active innate immune system. The pet store mice had higher baseline levels of interleukin-4 and -13 than their lab-grown counterparts and unsurprisingly, were more resilient to spiB induced ENS damage. Having some dirt around is good after all, at least for the mice!!
Why I chose this preprint?
The ongoing global pandemic has drawn our attention towards immunity and hygiene. There is no doubt that keeping good hygiene is essential to keep many infections at bay. On the other hand, an alternative notion was proposed by D.P Strachan in 1989, who suggested that being exposed to pathogens early in life is a good thing, as it helps to build immunity and reduces autoimmune disorders. It is a stretch to make such a blanket claim about hygiene being the cause of autoimmunity, but , research spanning decades have pointed towards a positive role of mutualistic and commensal microflora in bolstering our immune system. Helminth worms, although parasitic, have been demonstrated to have a positive role in reducing allergic reactions and autoimmune disorders [6]. This preprint by Ahrends et al., [4] sheds new light on the protection that helminths can offer against nerve damage caused by a bacterial pathogen. Additionally, the study has generated elegant genetic, pharmacological and immunomodulatory tools to dissect immune pathways, which will provide a wealth of resources for future studies.
References :
- Liu, Y., and Chen, Y.G. (2020). Intestinal epithelial plasticity and regeneration via cell dedifferentiation. Cell Regen 9, 14.
- Obermayr, F., and Seitz, G. (2018). Recent developments in cell-based ENS regeneration – a short review. Innov Surg Sci 3, 93-99.
- Matheis, F., Muller, P.A., Graves, C.L., Gabanyi, I., Kerner, Z.J., Costa-Borges, D., Ahrends, T., Rosenstiel, P., and Mucida, D. (2020). Adrenergic Signaling in Muscularis Macrophages Limits Infection-Induced Neuronal Loss. Cell 180, 64-78 e16.
- Ahrends, T., Aydin, B., Matheis, F., Classon, C., Furtado, G.C., Lira, S.A., and Mucida, D. (2021). Enteric pathogens induce tissue tolerance and prevent neuronal loss from subsequent infections. bioRxiv, 2021.2004.2009.439221.
- Lloyd, C.M., and Snelgrove, R.J. (2018). Type 2 immunity: Expanding our view. Sci Immunol 3.
- Stiemsma, L.T., Reynolds, L.A., Turvey, S.E., and Finlay, B.B. (2015). The hygiene hypothesis: current perspectives and future therapies. Immunotargets Ther 4, 143-157.
doi: Pending
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the immunology category:
A Novel Chimeric Antigen Receptor (CAR) - Strategy to Target EGFRVIII-Mutated Glioblastoma Cells via Macrophages
Dina Kabbara
Loss of MGST1 during fibroblast differentiation enhances vulnerability to oxidative stress in human heart failure
Jeny Jose
Scalable transcription factor mapping uncovers the regulatory dynamics of natural and synthetic transcription factors in human T cell states
Inês Caiado
Also in the microbiology category:
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Microbial Feast or Famine: dietary carbohydrate composition and gut microbiota metabolic function
Jasmine Talevi
Citrobacter rodentium infection activates colonic lamina propria group 2 innate lymphoid cells
André Luiz Amorim Costa, Marcus Oliveira
Also in the neuroscience category:
PPARδ activation in microglia drives a transcriptional response that primes phagocytic function while countering inflammatory activation
Isabel Paine
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Self-renewal of neuronal mitochondria through asymmetric division
Lorena Olifiers
Also in the pathology category:
Snake venom metalloproteinases are predominantly responsible for the cytotoxic effects of certain African viper venoms
Daniel Osorno Valencia
Schistosoma haematobium DNA and Eggs in the Urine Sample of School-Age Children (SAC) in South-West Nigeria
Hala Taha
FUS Mislocalization Rewires a Cortical Gene Network to Drive 2 Cognitive and Behavioral Impairment in ALS
Taylor Stolberg
preLists in the immunology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |
Also in the microbiology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |
Also in the neuroscience category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |
Also in the pathology category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
COVID-19 / SARS-CoV-2 preprints
List of important preprints dealing with the ongoing coronavirus outbreak. See http://covidpreprints.com for additional resources and timeline, and https://connect.biorxiv.org/relate/content/181 for full list of bioRxiv and medRxiv preprints on this topic
| List by | Dey Lab, Zhang-He Goh |
1
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |











 (No Ratings Yet)
(No Ratings Yet)