Bacterial filamentation is an in vivo mechanism for cell-to-cell spreading
Posted on: 30 January 2022 , updated on: 13 February 2022
Preprint posted on 21 November 2021
Article now published in Nature Communications at http://dx.doi.org/10.1038/s41467-022-28297-6
Skilful pathogens: a bacterium that forms filaments to colonise its nematode host
Selected by Angika BasantCategories: cell biology, ecology, pathology
How do pathogens spread from cell to cell in a live animal? What strategies do they use to navigate complex 3D environments and defence mechanisms in their host? There are few physiological contexts where these questions can be easily addressed as in vivo imaging of internal tissues is challenging in most organisms. However, nematodes are emerging as a useful model system to study infections in this regard.
Studies in 2D cell monolayers have shown that several species of bacteria usurp the host actin cytoskeleton to propel themselves into neighbouring cells either via lateral cell membranes or via filopodia extensions. In this preprint Tran et al. describe a new mode of bacterial spread that appears to avoid the risk of extracellular exposure of the pathogen in a unique fashion. The authors discover a bacterial pathogen in the nematode Oscheius tipulae that adopts a filamentous form to spread across host intestinal cells.
Key findings:
The authors isolated a wild Oscheius tipulae nematode strain from rotting crab apples. The worms contained coccobacilli-shaped microbes in their intestinal epithelia. These microbes were originally believed to be microsporidia. However, using a panel of microsporidia- and bacteria-specific rRNA in situ hybridization probes, the authors demonstrate that the pathogen in question is actually a bacterium. It could be isolated on LB agar plates and was determined to be a new species in a clade of Bordetella which the authors name Bordetella atropi. Interestingly, staging the infection cycle in worms by pulse-chase experiments revealed the presence of short and long intracellular bacterial filaments at 16- and 24-hours post-infection (hpi) whereas by 38 and 48 hpi most worms contained only the coccobacilli form.
Filaments up to 50 μm in length were observed by confocal microscopy. More detailed analysis with transmission electron microscopy shows nucleoids that appear to be dividing and possible septum formation within these filaments. Using fluorescent dyes that mark the cytoplasm of intestinal cells and actin markers that define the apical and basolateral cell edges, the authors confirm that Bordetella atropi is indeed an intracellular pathogen. It appears to first form filaments in intestinal epithelial cells which septate into coccobacilli prior to cell exit.
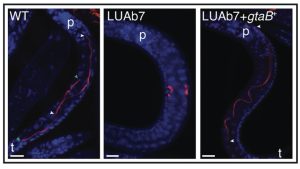
The authors next isolated a mutant variant of B. atropi, LUAb7 that is incapable of making filaments in vitro. Strikingly, when infected into the nematode host, LUAb7 showed decreased anterior-posterior spreading and primarily generated coccobacilli. The infection events that were filaments were reduced to 9% compared to 95% in the wildtype strain. Furthermore, WT B. atropi filaments spread to an average of 3 cells and maximum of 8 cells at 34 hpi, whereas LUAb7 was generally seen in a single cell and occasionally in two cells.
The causative mutation in LUAb7 turned out to be a missense mutation in gtaB, a UTP–glucose-1-phosphate uridylyltransferase. GtaB (known as GalU in E. coli) catalyzes glucose-1-phosphate to UDP-glucose conversion, which is required for cell wall synthesis. The R17C mutation identified in LUAb7 modifies a predicted catalytic arginine in a conserved N-terminal motif. Importantly, complementing LUAb7 with gtaB+ from B. atropi resulted in a rescue of in vitro and in vivo filamentation and cell-to-cell spread in the host. Knocking out other members of the UDP-glucose synthesis pathway in B. atropi revealed potential positive and negative regulators of the filamentation process during infection.
What I like about this preprint:
Both the host-pathogen model system used, and the identified mechanism of cell-to-cell spread are exciting and unique. I also like that the study includes the isolation of a filamentation-defective mutant that points to a metabolic pathway and molecular players that could regulate this process of bacterial spread. This opens many new avenues of investigation.
Questions for the authors:
- How do the bacteria span across membranes? What is the membrane topology at sites where the bacteria appear to “pierce” cell-cell junctions?
- Is there actin accumulating on coccobacilli? Maybe to facilitate cytosolic motility or are they likely to be non-motile?
- Were any events observed that point to the mechanism of cell exit by the coccobacilli? Is there a technical limitation on how many hours post-infection the infected worms can be imaged and monitored?
- LuAb7 reduces host fitness to the same extent as WT bacteria. In what way is the loss in the ability of cell-to-cell spread detrimental to LuAb7? Are the total number of produced bacteria/division cycles reduced during infection?
doi: https://doi.org/10.1242/prelights.31322
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
Cell cycle-dependent mRNA localization in P-bodies
Mohammed JALLOH
Control of Inflammatory Response by Tissue Microenvironment
Roberto Amadio
Notch3 is a genetic modifier of NODAL signalling for patterning asymmetry during mouse heart looping
Bhaval Parmar
Also in the ecology category:
Gestational exposure to high heat-humidity conditions impairs mouse embryonic development
Girish Kale, preLights peer support
Blue appendages and temperature acclimation increase survival during acute heat stress in the upside-down jellyfish, Cassiopea xamachana
Maitri Manjunath
How the liver contributes to stomach warming in the endothermic white shark Carcharodon carcharias
Sarah Young-Veenstra
Also in the pathology category:
LINC complex alterations are a hallmark of sporadic and familial ALS/FTD
Megane Rayer et al.
Hypoxia blunts angiogenic signaling and upregulates the antioxidant system in elephant seal endothelial cells
Sarah Young-Veenstra
H2O2 sulfenylates CHE linking local infection to establishment of systemic acquired resistance
Marc Somssich
preLists in the cell biology category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |
Also in the ecology category:
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
EMBO | EMBL Symposium: The organism and its environment
This preList contains preprints discussed during the 'EMBO | EMBL Symposium: The organism and its environment', organised at EMBL Heidelberg, Germany (May 2023).
| List by | Girish Kale |
Bats
A list of preprints dealing with the ecology, evolution and behavior of bats
| List by | Baheerathan Murugavel |
Also in the pathology category:
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
COVID-19 / SARS-CoV-2 preprints
List of important preprints dealing with the ongoing coronavirus outbreak. See http://covidpreprints.com for additional resources and timeline, and https://connect.biorxiv.org/relate/content/181 for full list of bioRxiv and medRxiv preprints on this topic
| List by | Dey Lab, Zhang-He Goh |
1
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |











 (No Ratings Yet)
(No Ratings Yet)