Members of the Arabidopsis auxin receptor gene family are essential early in embryogenesis and have broadly overlapping functions
Posted on: 4 April 2019
Preprint posted on 23 January 2019
Article now published in eLife at http://dx.doi.org/10.7554/eLife.54740
Auxin receptor TIR1/AFB is a happy family of genes that work together in plant embryogenesis.
Selected by Chandra Shekhar MisraCategories: developmental biology, plant biology
Introduction
Auxin is one of the most important phytohormones involved in cell differentiation, root and shoot development; it mediates plant responses to both abiotic and biotic stresses [1]. Auxin regulation of gene expression is dependent on three families of proteins: Auxin Response Factors (ARF), Aux/IAA transcriptional repressors and Transport Inhibitor Response 1 (TIR1)/Auxin signalling F-Box (AFB) proteins [1-2].
The Arabidopsis genome encodes six TIR1/AFB proteins that arise out of whole-genome duplication and each of these proteins contain an amino-terminal F-Box followed by Leucine-rich repeats (LRRs) (Fig. 1). The authors argue that duplication and diversification of these three gene families might be the reason auxin acquired a new possible role in vascular development, lateral root formation, and organ polarity. Though previous studies have revealed functions of individual members of this gene family, a comprehensive study about this auxin gene family has been lacking. Authors in this study have tried to unravel the role of the TIR1/AFB gene family in Arabidopsis that led them to uncover the functional redundancy of this family in their genetic analysis. The authors also identified a previously unknown function of this gene family in early embryogenesis.
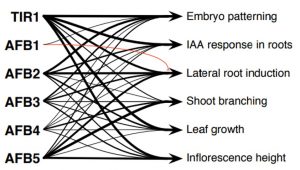
Fig 1: Role of six TIR1/AFB gene family in Arabidopsis (With due permission from Prigge et al., 2019, bioRxiv 529248)
Key findings
The authors did a phylogenetic analysis and found that the TIR1/AFB gene family had diversified before ferns-seeds-plants, over 400 million years ago. However, it was astonishing that despite this long divergence, TIR/AFB proteins retained largely similar functions. Though the authors found TIR1 to be the most important member of this family, AFB5, AFB2 followed by AFB3 and AFB4 also played a significant role.
The authors also identified tissue-specific expression patterns of different members of this gene family. For instance, they found TIR1 and AFB2 to be expressed more in the root, compared to AFB5 which was expressed at high level in the inflorescence. The authors found no to little expression of the AFB4 gene in all tissues, concluding that it played a very minor role in the gene family.
The authors also found that AFB1 uniquely differs from the rest of the TIR1/AFB members. Unlike all other members, AFB1 is expressed at a very high level in the root epidermis and vascular tissues and is absent from any other tissue. Genetic studies further revealed that AFB1 had no effect on root elongation, but surprisingly it was found to be a negative regulator of lateral root formation.
The authors found that most members of this family from TIR1 to AFB2 to AFB5 are all localized to the nucleus, but some proteins were also found to be present in the cytoplasm, for reasons which remain unknown.
The authors finally explored the importance of auxin in embryo patterning, a function lately well described in the literature [3]. The authors found that in a tir1afb235 quadruple mutant, apical cells and hypophysis suffered cell division errors (Fig. 2). Embryos from tir1afb235 quadruple and tir1afb12345 sextuple mutants were fully lethal.
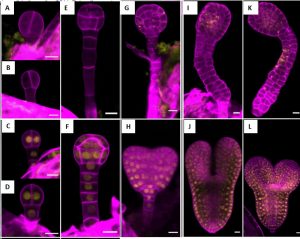
Fig 2: Representation of embryo-lethal phenotypes of tir1/afb sextuple mutant characterised by absence of cotyledon primordia and over-proliferated suspensors in some of the embryo development stages. The embryo stages are 2-cell (A–D), 16- cell (dermatogen) (E-F), late transition (G–H), torpedo (I-–J), and bent cotyledon (K–L). Upper panel shows the mutants with absence of mCitrine signal (Yellow) while those in the lower panel are complemented siblings showing the presence of citrine signal. (With due permission from Prigge et al., 2019, bioRxiv 529248)
The authors also noted that no embryonic markers were expressed in the quadruple and sextuple mutant which led to the defects in embryo patterning. Although tir1afb235 quadruple mutant formed a normal hypophysis, they never showed the expression of embryonic markers such as NTT (NO TRANSMITTING TRACT) or WOX5. Suspensor marker PIN7 was found to have reduced expression in the suspensor which led to its proliferation, however, it was also found to be expressed in the basal half of the embryo which led the authors to hypothesize that PIN7 expression in the embryo might be linked to the TIR1/AFB pathway.
What I liked about this preprint
Auxin is an important plant hormone responsible for plant growth and development. This hormone regulates gene expression which is dependent on three families of proteins as described above [4]. While some studies have described the role of this gene regulatory network on auxin-mediated responses, detailed analysis of this gene family was lacking. The authors have very clearly identified the role of six TIR1/AFB proteins and established that they share overlapping functions. However, what was most interesting was the role of this gene family in embryogenesis. Though several auxin mutants have been reported before with varying defects, the authors in this study found the embryos to be completely lethal in quadruple and sextuple mutants.
Questions for the authors
- The authors have alluded to the role of TIR1/AFBs under specific conditions. Since auxin is known to play an important role in various abiotic and biotic stress responses, did they check the function of these genes in other environmental conditions?
- It would be also interesting to know whether the authors have checked what is the effect of exogenous IAA specifically in the sextuple mutant tir1afb12345?
- Can the authors explain the possible underlying mechanism of this complex, as it is clear that although the gene family has overlapping functions, it looks like TIR1 or AFB2 alone is enough to rescue the growth. Can they be called a master regulator of this complex ?
References
- Zhao, Y., 2010. Auxin biosynthesis and its role in plant development. Annual review of plant biology, 61, pp.49-64.
- Lavy, M. and Estelle, M., 2016. Mechanisms of auxin signaling. Development, 143(18), pp.3226-3229.
- Palovaara, J., de Zeeuw, T. and Weijers, D., 2016. Tissue and organ initiation in the plant embryo: a first time for everything. Annual review of cell and developmental biology, 32, pp.47-75.
- Dindas, J., Scherzer, S., Roelfsema, M.R.G., Meyer, K., Müller, H.M., Al-Rasheid, K.A.S., Palme, K., Dietrich, P., Becker, D., Bennett, M.J. and Hedrich, R., 2018. AUX1-mediated root hair auxin influx governs SCF TIR1/AFB-type Ca 2+ signaling. Nature communications, 9(1), p.1174.
doi: https://doi.org/10.1242/prelights.9800
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the developmental biology category:
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
Dosage-sensitive RBFOX2 autoregulation promotes cardiomyocyte differentiation by maturing the transcriptome
Theodora Stougiannou
Post-translational Tuning of Human Cortical Progenitor Neuronal Output
Jawdat Sandakly
Also in the plant biology category:
Actin Counters Geometry to Guide Plant Cell Division
Jeny Jose
The nucleus follows an internal cellular scale during polarized root hair cell development
Jeny Jose
Conservation and divergence of regulatory architecture in nitrate-responsive plant gene circuits
Jeny Jose
preLists in the developmental biology category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
BSDB/GenSoc Spring Meeting 2024
A list of preprints highlighted at the British Society for Developmental Biology and Genetics Society joint Spring meeting 2024 at Warwick, UK.
| List by | Joyce Yu, Katherine Brown |
GfE/ DSDB meeting 2024
This preList highlights the preprints discussed at the 2024 joint German and Dutch developmental biology societies meeting that took place in March 2024 in Osnabrück, Germany.
| List by | Joyce Yu |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
2nd Conference of the Visegrád Group Society for Developmental Biology
Preprints from the 2nd Conference of the Visegrád Group Society for Developmental Biology (2-5 September, 2021, Szeged, Hungary)
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Society for Developmental Biology 79th Annual Meeting
Preprints at SDB 2020
| List by | Irepan Salvador-Martinez, Martin Estermann |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EDBC Alicante 2019
Preprints presented at the European Developmental Biology Congress (EDBC) in Alicante, October 23-26 2019.
| List by | Sergio Menchero et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |
Pattern formation during development
The aim of this preList is to integrate results about the mechanisms that govern patterning during development, from genes implicated in the processes to theoritical models of pattern formation in nature.
| List by | Alexa Sadier |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |
Also in the plant biology category:
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |











 (No Ratings Yet)
(No Ratings Yet)