A Bile Duct-on-a-Chip with Organ-Level Functions
Posted on: 19 April 2019 , updated on: 29 September 2019
Preprint posted on 30 March 2019
Article now published in Hepatology at http://dx.doi.org/10.1002/hep.30918
Categories: bioengineering, pharmacology and toxicology
Background of preprint
Alterations in the tight junctions of bile duct epithelial cells, or cholangiocytes, have been associated with chronic cholestatic liver diseases both in humans and in mouse models. However, most in vitro biliary research currently involves the use of cultured cells in either 2D monolayers or 3D organoids, both of which are not representative of bile duct structural organisation and cannot perform biliary physiological functions like compartmentalising bile. To bridge this gap between biliary physiology and existing biliary models, Du et al. apply organ-on-chip technology to develop a micro-engineered bile duct. As described by the authors, the significance of this is threefold:
- The bile duct-on-a-chip performs key functions of the bile duct,
- The bile duct-on-a-chip is a useful tool that can be used to study the barrier function of the cholangiocyte monolayer quantitatively, and
- The barrier function of the cholangiocyte monolayer at the apical or basolateral side can be studied independently.
Finally, the authors provide a proof-of-concept by using their invention to demonstrate the protective role of the cholangiocyte apical glycocalyx.
Key findings of preprint
The findings of this preprint can be categorised into four main sections (Fig. 1). First, the authors fabricated and characterised the bile duct-on-a-chip. Second, Du et al. proved that independent access to the apical and basal surfaces of the cholangiocyte monolayer could be achieved in their invention. Third, the authors used the bile duct-on-a-chip to show that the glycocalyx protects cholangiocytes from bile acid-induced damage. Fourth, the authors demonstrated that their device could be used with cells from other sources.
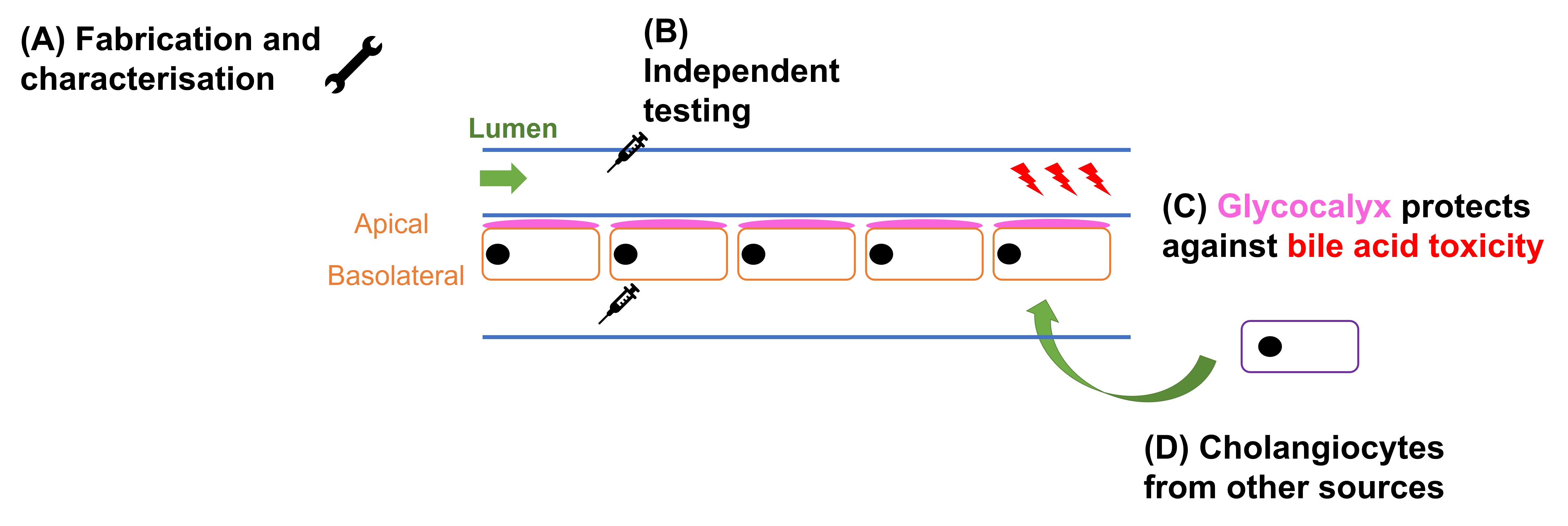
Figure 1. Four key findings in the preprint by Du et al.
(A) Fabrication and characterisation of bile duct-on-a-chip
Du et al. first developed the bile duct-on-a-chip by lining a channel with mouse cholangiocytes, which formed a confluent and compact epithelial monolayer as shown using F-actin staining. Characterising these cholangiocytes yielded four major observations:
- These cholangiocytes maintained the expression of the cholangiocyte marker K19.
- The expression of ZO-1 and E-cadherin 1 at cell-cell junctions demonstrated the formation of tight junctions.
- ASBT staining confirmed the polarisation of cells on the bile duct-on-a-chip.
- Cholangiocytes had to be superconfluent and compact in order to give rise to full barrier function with a significant decrease in permeability. To demonstrate that the tight junction formation indeed led to the barrier function of the bile duct, the authors perfused the lumen with a range of sizes of FITC-dextran (4-70 kDa) and showed that there was no clear leakage of this fluorescent dextran into the collagen matrix after 10 minutes.
(B) Apical and basal surfaces of the cholangiocyte monolayer can be accessed independently
To show that the apical and basal surfaces of the cholangiocyte monolayer react differently to different toxins, Du et al. applied both the toxic isoflavonoid biliatresone [1] and the bile acid glycochenodeoxycholic acid (GCDC) independently to each side of the bile duct-on-a-chip. The authors found that this indeed increased the permeability of monolayers in the bile duct-on-a-chip. Furthermore, this damage was worse with basal than apical administration, an observation ascribed to the lower tolerance of bile from the basolateral side [2-4]. Based on this finding, the authors posited that leakage of toxic bile through the epithelial monolayer can give rise to a feedback loop that results in increasingly severe toxicity.
(C) Proof-of-concept: The apical glycocalyx protects cholangiocytes from bile acid-induced damage
Du et al. then used their invention to show that demonstrate the cholangiocyte-lined channel is resistant to bile acid toxicity. This resistance could be attributed to the apical glycocalyx, which is known to protect against bile acid toxicity [5,6]—this protective effect against GCDC was lost after removal with neuraminidase, and the permeability of the cholangiocyte-lined channels towards 4 and 10 kDa (but not 70 kDa) FITC-dextran increased.
(D) Demonstration of compatibility of bile duct-on-a-chip with cells from other sources
When Du et al. seeded and characterised their device using primary murine extrahepatic cholangiocytes via a similar procedure, they found that the cholangiocytes in the device (a) expressed K19, (b) exhibited cell junctions that were even tighter than those previously investigated, and (c) exhibited polarisation.
Significance of this preprint: Current developments and future directions
Before the invention of the first organ-on-a-chip a decade ago [7], the idea may have sounded—to borrow the words from Miguel Rivera from Pixar’s Coco—un poco loco (a little crazy). But the technology has since come a long way. Today, organs-on-a-chip are highly valuable miniaturisation technologies that can fill many roles. The same can be said about the bile duct-on-a-chip invented by preprint authors. It adds to a veritable trove of bioengineered bile ducts [8,9]. As the authors point out in their preprint, the device has four distinctive benefits:
- Two sets of ports that enable sampling of luminal contents,
- Independent and selective exposure of the apical and basolateral sides to drugs or toxins,
- Variation of the chemical composition and mechanics of the surrounding matrix, and
- Variation of the fluid flow rate.
The potential applications of the bile duct-on-a-chip are many. It could be used to further explore the hepatobiliary tree, whose complex environment makes the pathophysiology of associated diseases particularly baffling. In pharmacokinetic testing phases of drug discovery, too, the bile duct-on-a-chip could prove useful: the hepatobiliary system plays a crucial role in drug disposition, and a validated model of a bile duct-on-a-chip could open a host of exciting in vitro pharmaceutical biotesting applications. The bile duct-on-a-chip could even be used to understand the hepatobiliary system more holistically, which would aid in efforts to characterise how drugs and toxins might affect the entire hepatobiliary system.
Questions for authors
- What are some potential reasons for the better barrier function demonstrated by the primary cell-lined device compared to the cholangiocyte cell line? What might be the physiological significance of this difference?
- What are some challenges in biliary pathophysiology that you hope to solve using this device?
References
[1] Waisbourd‐Zinman O, Koh H, Tsai S, Lavrut PM, Dang C, Zhao X, Pack M, Cave J, Hawes M, Koo KA, The toxin biliatresone causes mouse extrahepatic cholangiocyte damage and fibrosis through decreased glutathione and SOX17, Hepatology 64(3) (2016) 880-893.
[2] Hopkins AM, Walsh SV, Verkade P, Boquet P, Nusrat A, Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function, Journal of cell science 116(4) (2003) 725-742.
[3] Xia X, Francis H, Glaser S, Alpini G, LeSage G, Bile acid interactions with cholangiocytes, World journal of gastroenterology: WJG 12(22) (2006) 3553.
[4] Benedetti A, Alvaro D, Bassotti C, Gigliozzi A, Ferretti G, La Rosa T, Di Sario A, Baiocchi L, Jezequel AM, Cytotoxicity of bile salts against biliary epithelium: a study in isolated bile ductule fragments and isolated perfused rat liver, Hepatology 26(1) (1997) 9-21.
[5] Hohenester S, Maillette de Buy Wenniger L, Paulusma CC, van Vliet SJ, Jefferson DM, Oude Elferink RP, Beuers U, A biliary HCO3− umbrella constitutes a protective mechanism against bile acid‐induced injury in human cholangiocytes, Hepatology 55(1) (2012) 173-183.
[6] de Buy Wenniger LJM, Hohenester S, Maroni L, van Vliet SJ, Elferink RPO, Beuers U, The Cholangiocyte Glycocalyx Stabilizes the ‘Biliary HCO3-Umbrella’: An Integrated Line of Defense against Toxic Bile Acids, Digestive Diseases 33(3) (2015) 397-407.
[7] Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE, Reconstituting Organ-Level Lung Functions on a Chip, Science 328(5986) (2010) 1662-1668.
[8] Sampaziotis F, Justin AW, Tysoe OC, Sawiak S, Godfrey EM, Upponi SS, Gieseck III RL, de Brito MC, Berntsen NL, Gómez-Vázquez MJ, Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids, Nature medicine 23(8) (2017) 954.
[9] Chen C, Jochems PG, Salz L, Schneeberger K, Penning LC, Van De Graaf SF, Beuers U, Clevers H, Geijsen N, Masereeuw R, Bioengineered bile ducts recapitulate key cholangiocyte functions, Biofabrication 10(3) (2018) 034103.
doi: https://doi.org/10.1242/prelights.10255
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the bioengineering category:
A Novel Chimeric Antigen Receptor (CAR) - Strategy to Target EGFRVIII-Mutated Glioblastoma Cells via Macrophages
Dina Kabbara
Human pluripotent stem cell-derived macrophages modify development of human kidney organoids
Theodora Stougiannou
Matrix viscoelasticity regulates dendritic cell migration and immune priming
Roberto Amadio
Also in the pharmacology and toxicology category:
Snake venom metalloproteinases are predominantly responsible for the cytotoxic effects of certain African viper venoms
Daniel Osorno Valencia
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
In vitro pharmacokinetics and pharmacodynamics of the diarylquinoline TBAJ-587 and its metabolites against Mycobacterium tuberculosis
Zhang-He Goh
preLists in the bioengineering category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Advances in microscopy
This preList highlights exciting unpublished preprint articles describing advances in microscopy with a focus on light-sheet microscopy.
| List by | Stephan Daetwyler |
Also in the pharmacology and toxicology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
COVID-19 / SARS-CoV-2 preprints
List of important preprints dealing with the ongoing coronavirus outbreak. See http://covidpreprints.com for additional resources and timeline, and https://connect.biorxiv.org/relate/content/181 for full list of bioRxiv and medRxiv preprints on this topic
| List by | Dey Lab, Zhang-He Goh |
1
Drug use in special populations
Any drugs that are being used in special populations: Patients with liver and kidney failure, in paediatrics, in geriatrics, and in pregnant or lactating patients. Includes the discovery of factors that could potentially affect drug use in these special populations.
| List by | Zhang-He Goh |
Toxicology of toxicants, existing therapeutics, and investigational drugs
Preprints that describe the toxicology of environmental pollutants and existing and upcoming drugs. Includes both toxicokinetics and toxicodynamics, as well as technological improvements that will help in the characterisation of this field.
| List by | Zhang-He Goh |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |
Anticancer agents: Discovery and clinical use
Preprints that describe the discovery of anticancer agents and their clinical use. Includes both small molecules and macromolecules like biologics.
| List by | Zhang-He Goh |
Advances in Drug Delivery
Advances in formulation technology or targeted delivery methods that describe or develop the distribution of small molecules or large macromolecules to specific parts of the body.
| List by | Zhang-He Goh |











 (No Ratings Yet)
(No Ratings Yet)