A synthetic mechanogenetic gene circuit for autonomous drug delivery in engineered tissues
Posted on: 6 May 2020
Preprint posted on 1 May 2020
Article now published in Science Advances at http://dx.doi.org/10.1126/sciadv.abd9858
You’ll be in my joint: gene circuits in engineered cartilage tissue constructs for autonomous drug delivery
Selected by Zhang-He GohCategories: bioengineering, pharmacology and toxicology, synthetic biology
Background of preprint: take my hand, ice it right
Osteoarthritis is one of the most prevalent chronic joint diseases worldwide. Arising from repeated stresses on the joints, the prevalence of osteoarthritis can reach 50% in older populations [1]. Patients with osteoarthritis can experience significant amounts of inconvenience and pain, and osteoarthritis can severely limit patients’ quality of life, especially as a co-morbidity with other age-related diseases, such as frailty. It is therefore unsurprising that the prevalence of osteoarthritis among ageing populations is gaining global attention, especially in countries with large ageing populations.
The current standard of care in osteoarthritis largely rests on the twin pillars of both pharmacological and non-pharmacological treatment. Pharmacologic intervention includes the use of painkillers, including anti-inflammatory agents, like paracetamol, ibuprofen, and naproxen. Non-pharmacological advice usually revolves around frequent resting of joints, and the application of topical aids like ice packs. Surgical interventions may be used as a last resort where indicated.
In response to these limits of medical intervention, Nims et al. engineered a mechanically responsive bioartificial tissue construct for therapeutic drug delivery (Fig. 1). They utilise a mechano-osmosensitive member of the family of transient receptor channels (TRP)—TRPV4—to drive synthetic mechanogenetic circuits. This ultimately results in the production of an anti-inflammatory biologic, interleukin-1 receptor antagonist (IL-1Ra).
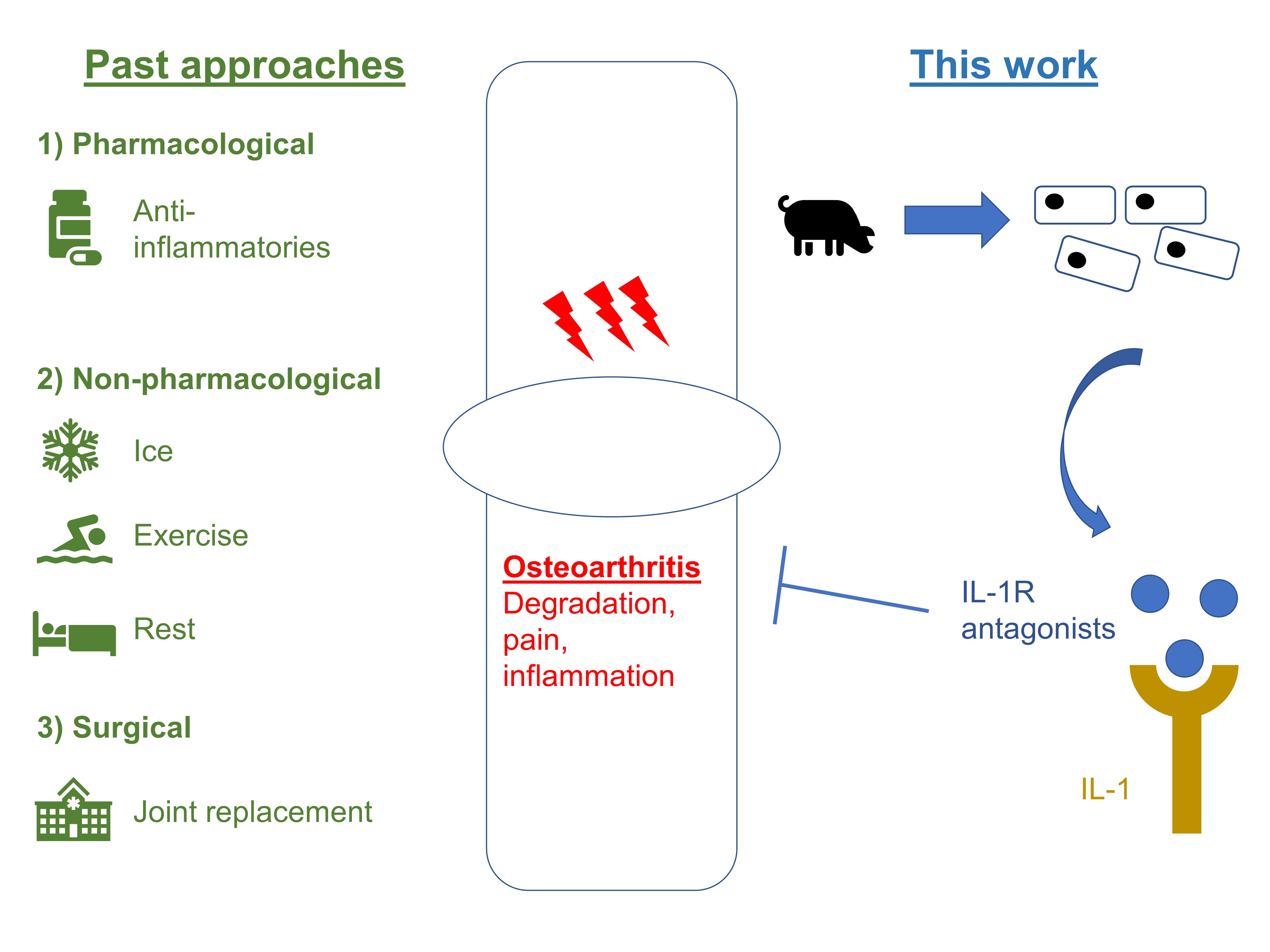
Figure 1. Relation of this work to current standards of care.
Key findings of preprint: what we can explain (and engineer)
The findings of this preprint can be broadly classified into three categories.
First, the authors established that mechanical loading of tissue-engineered cartilage activates TRPV4 through fluctuations in the local osmotic environment, rather than direct mechanical deformation of chondrocytes. Using computational modelling and experimental methods, Nims et al. showed that membrane deformation alone did not induce calcium signalling responses in chondrocytes; the same computational model showed that osmotic fluctuations could induce signalling in chondrocytes.
Second, the authors elucidated the mechanosensitive transcription factors downstream of TRPV4 activation. Based on this series of experiments, the authors concluded that the TRPV4 mechanotransduction pathway involves the activation of inflammatory pathway responses. This knowledge was then used to engineer synthetic gene circuits which would respond to mechanical TRPV4 activation by driving the production of IL-1Ra. Specifically, the authors used lentiviral systems to produce synthetically-programmed cartilage constructs from primary porcine chondrocytes.
Third, the authors demonstrated how these engineered tissue constructs induced an autonomous mechanogenetic response that protected tissues from inflammatory insults. Having established that their new construct functioned over a range of conditions, Nims et al. then tested the response of these engineered constructs to IL-1α, as well as the ability of the produced IL-1Ra to protect surrounding tissue against IL-1α. The former was tested using an exogenous source of IL-1α; the latter was tested using a co-culture method, and the loss of sulphated-glycosaminoglycan content (S-GAG) was measured. Nims et al. found that their engineered constructs produced IL-1Ra in response to exogenous IL-1α, and also protected against S-GAG loss.
What I like about this preprint: one so small and so strong
Nims et al. have “developed a novel class of mechanogenetically-sensitive bioartificial material”. The authors’ technology potentially “(obviates) many of the traditional limitations of ‘smart’ materials, such as long-term integration, rapid dynamic responses, and extended drug delivery without the need for replacement or reimplantation of the drug delivery system”. The bioartificial material developed by Nims et al. is interesting from the perspective that it uses a living tissue construct for drug delivery, albeit a non-human one.
In the literature, successful implementation of the direct implant of tissues to perform the dual role of being both the source and the mode of delivery of the biologic is still in its infancy; most research to date has been devoted to bioengineered tissues for other medical applications like skin grafting.
Future directions: will you be in my joint?
As Nims et al. point out, their technology is currently a proof-of-concept, so much more research will need to be performed in realising the authors’ final goal of implanting this in patients with osteoarthritis.
Three main challenges lie ahead. Two of these are associated with the use of a porcine cell source, and the third relates to further in vivo studies.
The first challenge is scientific: the immunogenicity of the porcine chondrocytes that may result in rejection of the construct by the host will be an important consideration. To paraphrase Tarzan: the constructs will be deep inside us. The question is, how different are they from us? The host cells may produce antibodies that reject the tissue construct, a risk that is higher given that cells from a different species are used—the construct utilises cells derived from a porcine (rather than a human) source.
The second challenge is cultural. Patients in certain cultures or religions may be more cognizant of the sources of certain medical products. It may be necessary, for example, to derive gelatin from bovines or plants, rather than the usual porcine sources. Similar considerations may apply to the use of these sources in the synthesis of these tissue constructs.
The third challenge is medical. In vivo studies will need to be conducted to complement the in vitro studies reported in this preprint. While these constructs are unlikely to pose significant toxicity risks, their efficacy in responding to mechanical stresses (or, more specifically, osmotic changes) in the joint still needs to be demonstrated before moving the technology into clinical trials.
Notwithstanding these challenges that face researchers in moving this construct into medical practice, Nims et al. have presented an interesting way of using genetic modification to exploit cells’ mechanosensing abilities in biologic production and delivery. The applications of this technology may well extend beyond therapeutics into molecular probes, medical diagnostics, and even bioengineering. Much work and promise lie ahead.
Questions for authors
- Why were primary porcine chondrocytes used to create the synthetically-programmed cartilage constructs? What advantages might these primary chondrocytes offer over other immortalised cell lines, such as immortal human chondrocytes?
References
[1] Castell MV, van der Pas S, Otero A, Siviero P, Dennison E, Denkinger M, Pedersen N, Sanchez-Martinez M, Queipo R, van Schoor N, Zambon S, Edwards M, Peter R, Schaap L, Deeg D, Osteoarthritis and frailty in elderly individuals across six European countries: results from the European Project on OSteoArthritis (EPOSA), BMC Musculoskeletal Disorders 16(1) (2015) 359.
doi: https://doi.org/10.1242/prelights.20154
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the bioengineering category:
A Novel Chimeric Antigen Receptor (CAR) - Strategy to Target EGFRVIII-Mutated Glioblastoma Cells via Macrophages
Dina Kabbara
Human pluripotent stem cell-derived macrophages modify development of human kidney organoids
Theodora Stougiannou
Matrix viscoelasticity regulates dendritic cell migration and immune priming
Roberto Amadio
Also in the pharmacology and toxicology category:
Snake venom metalloproteinases are predominantly responsible for the cytotoxic effects of certain African viper venoms
Daniel Osorno Valencia
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
In vitro pharmacokinetics and pharmacodynamics of the diarylquinoline TBAJ-587 and its metabolites against Mycobacterium tuberculosis
Zhang-He Goh
Also in the synthetic biology category:
Enzymatic bromination of native peptides for late-stage structural diversification via Suzuki-Miyaura coupling
Zhang-He Goh
Enhancer cooperativity can compensate for loss of activity over large genomic distances
Milan Antonovic
Discovery and Validation of Context-Dependent Synthetic Mammalian Promoters
Jessica L. Teo
preLists in the bioengineering category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Advances in microscopy
This preList highlights exciting unpublished preprint articles describing advances in microscopy with a focus on light-sheet microscopy.
| List by | Stephan Daetwyler |
Also in the pharmacology and toxicology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
COVID-19 / SARS-CoV-2 preprints
List of important preprints dealing with the ongoing coronavirus outbreak. See http://covidpreprints.com for additional resources and timeline, and https://connect.biorxiv.org/relate/content/181 for full list of bioRxiv and medRxiv preprints on this topic
| List by | Dey Lab, Zhang-He Goh |
1
Drug use in special populations
Any drugs that are being used in special populations: Patients with liver and kidney failure, in paediatrics, in geriatrics, and in pregnant or lactating patients. Includes the discovery of factors that could potentially affect drug use in these special populations.
| List by | Zhang-He Goh |
Toxicology of toxicants, existing therapeutics, and investigational drugs
Preprints that describe the toxicology of environmental pollutants and existing and upcoming drugs. Includes both toxicokinetics and toxicodynamics, as well as technological improvements that will help in the characterisation of this field.
| List by | Zhang-He Goh |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |
Anticancer agents: Discovery and clinical use
Preprints that describe the discovery of anticancer agents and their clinical use. Includes both small molecules and macromolecules like biologics.
| List by | Zhang-He Goh |
Advances in Drug Delivery
Advances in formulation technology or targeted delivery methods that describe or develop the distribution of small molecules or large macromolecules to specific parts of the body.
| List by | Zhang-He Goh |
Also in the synthetic biology category:
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |











 (No Ratings Yet)
(No Ratings Yet)