A unicellular relative of animals generates an epithelium-like cell layer by actomyosin-dependent cellularization
Posted on: 5 April 2019 , updated on: 9 November 2019
Preprint posted on 28 February 2019
Article now published in eLife at https://elifesciences.org/articles/49801
(Transiently) Comfortable in its own “skin”: formation of epithelium-like multicellular structures in a unicellular organism through conserved actomyosin-dependent mechanisms.
Selected by Paul Gerald L. Sanchez and Stefano VianelloCategories: developmental biology, evolutionary biology
Background
In multicellular organisms, cells arrange over time and space to give rise to tissues with distinct architectures and functional properties. Cells will arrange themselves as ordered arrays in epithelial tissues, they will be loose and sparse in connective tissues, they will give rise to even more distinctive architectures in muscle and nervous tissues. Of all these forms of organisations however, epithelia seem to hold a special place in biology. During early embryonic development of mammalian species, it is indeed as an epithelium that cells first arrange themselves when forming the blastocyst. It is still an epithelium that acts as the substrate of gastrulation in chicken, mouse, and humans. It is again to an epithelium that cells apparently default to when even just seeded within a matrix. Epithelia can fold, form sheets and tubes, and they can support morphogenesis. Epithelia have a barrier function and a polarity: they compartmentalise space and they can distinguish and generate differences between these compartments.
Motivating the work highlighted by this preLight, epithelia have been suggested to be the first form of multicellular organisation to arise during evolution. While their ubiquitous presence across all animals clearly makes them a characteristic feature of this group, the discovery of polarized animal-like epithelia in social amoebae raised the possibility that these structures might even pre-date animals, and as such reflect an even more ancestral cellular programme. Yet, the evolutionary branches separating animals from slime moulds are also populated by unicellular life forms: epithelial organisation might thus just be the result of convergent independent evolution of epithelial-like organisation from a non epithelial ancestor.
The authors here investigate an intermediate branch of the evolutionary space separating animals from slime-moulds (i.e. the ichthyosporeans branch), and beautifully describe here too an epithelial-like life stage. Even more interestingly, the cellularization process giving rise to such epithelium occurs via animal-like mechanisms, strengthening evolutionary models that see epithelial structures as a basal characteristic of not only animals but of all unikonts.
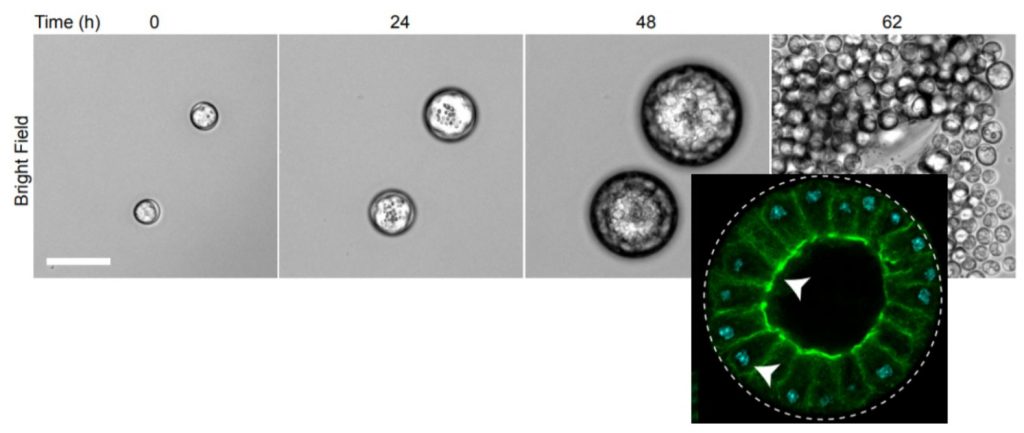
Life cycle of Sphaeroforma arctica. Inset: labeling for nucleus (DAPI, cyan) and actin (phalloidin, green), for a sample fixed just before the flip event, showing polarized nuclear localization and cellularization.
Key findings
The authors previously found that the single-cell ichthyosporean Sphaeroforma arctica transits through a multicellular stage [Ondracka et al, 2018]. They find that this stage displays features typical of an epithelium (Figure A). Such a transient epithelial-like structure originates by cellularization of a coenocyte, a shared multinucleated cytoplasm generated by rounds of cell division without cytokinesis.
By using a combination of membrane and cytoskeleton marking, live imaging, and RNA sequencing of cultured organisms, the authors find that this cellularization process occurs through animal-like mechanisms. Specifically, they find that it occurs through the stepwise assembly and deployment of a cortical network of actin and myosin (Figure B):
- nucleation of actin at the cortex,
- formation of filaments,
- myosin-dependent crosslinking,
- membrane invagination to compartmentalise the underlying cytoplasm
Concomitantly, the organism undergoes a drastic transcriptional shift to genes involved in cell-cell and cell-matrix interactions, as well as in cytoskeletal components. Morphologically, and mechanistically, the process is conserved with what is seen in animal models of cellularization (e.g. in Drosophila); yet also reveals ichthyosporean-specific features such as the role of transcriptional changes.
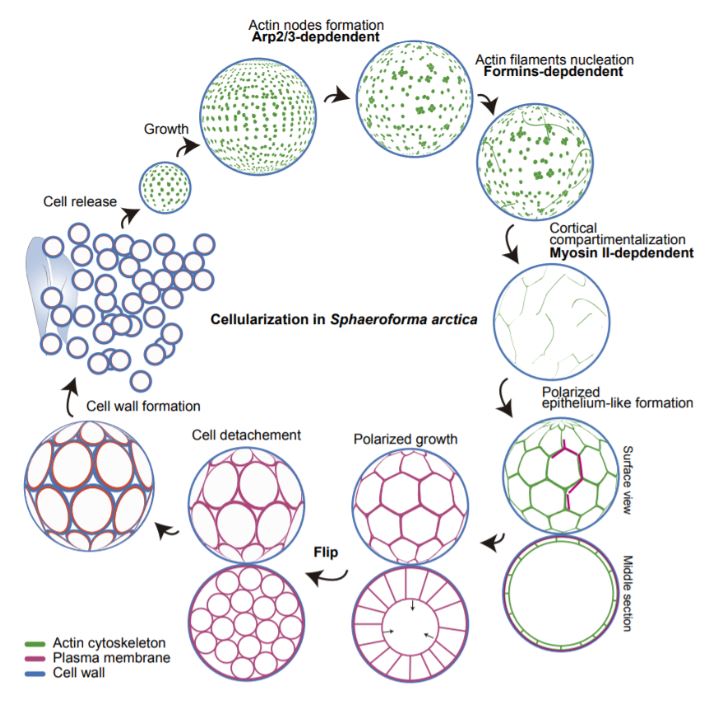
Summary of actomyosin-dependent cellularization in Sphaeroforma arctica.
Significance
This study identifies animal-like mechanisms of epithelial formation in yet another branch of the evolutionary tree. That is, in addition to that of the slime moulds, where the identification of catenin-based epithelial structures prompted hypothesis of a pre-animal emergence of epithelial programmes [Dickinson et al, 2012]. The S. arctica studied here (ichthyosporean) belongs to a lineage even closer to the animal branchpoint. While previous descriptions in slime moulds could have just been the result of independent evolution in slime moulds and animals, finding conserved molecular mechanisms in ichthyosporeans strengthens the hypothesis of common descent from an ancestor that did already have epithelial-like life stages. Such epithelial programmes would have been maintained in the animal, ichthyosporean, and slime-mould lineages, and lost in the many exclusively unicellular life-forms observed in between these branches.
We particularly appreciated how elegant the experimental design is, considering the limited tools available when studying an emerging model system. The authors maximized the amount of biological insight obtained from membrane/cytoskeleton labelling and targeted small molecule inhibition of different steps of cytoskeletal organisation. Of notice is also the RNA sequencing data collection, aligned to a genome re-assembled by the authors themselves!
Open questions
- You seem always very careful about talking about “epithelium-like” tissue, and not “epithelium”. Could you elaborate on this distinction? What features do you see as still missing to define this as an epithelium?
- Could you elaborate on the differences you see when comparing to Drosophila? What do you think these reveal about the requirements and evolutionary strategies of cellularization?
- What tools would you like to have available to prove the actual involvement of catenins? And to test the function of the epithelium?
- Animal epithelia rarely form through cellularization of a coenocyte. Could you elaborate on that? How widespread is epithelialisation (rather than cellularization) outside of the animal group?
- Kinesin 2 is one of the few microtubule motors that also undergoes upregulation upon cellularization. Do you have any ideas on whether it contributes to membrane folding too?
- Coenocyte sizes seem to vary during growth. Do bigger coenocytes take shorter time to burst after the flip event, explaining the observed variability? Do they release more/bigger cells?
Further reading
A comprehensive review on epithelia and their function:
- Rodriguez-Boulan, Enrique, and Ian G. Macara. “Organization and execution of the epithelial polarity programme.” Nature Reviews Molecular Cell Biology 15.4 (2014): 225.
Overview of the current evolutionary considerations on the origin of epithelia:
- Dickinson, Daniel J., W. James Nelson, and William I. Weis. “An epithelial tissue in Dictyostelium challenges the traditional origin of metazoan multicellularity.” Bioessays 34.10 (2012): 833-840.
A previous paper by the same lab as this preprint:
- Ondracka, Andrej, Omaya Dudin, and Iñaki Ruiz-Trillo. “Decoupling of Nuclear Division Cycles and Cell Size during the Coenocytic Growth of the Ichthyosporean Sphaeroforma arctica.” Current Biology 28.12 (2018): 1964-1969.
An article on the root as a polarized epithelium in plants:
- Maizel, Alexis. “Plant Biology: The Making of an Epithelium.” Current Biology 28.17 (2018): R931-951.
doi: https://doi.org/10.1242/prelights.9812
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the developmental biology category:
Tissue mechanics and systemic signaling safeguard epithelial tissue against spindle misorientation
Ruoheng Li
Human pluripotent stem cell-derived macrophages modify development of human kidney organoids
Theodora Stougiannou
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
Also in the evolutionary biology category:
Morphological variations in external genitalia do not explain the interspecific reproductive isolation in Nasonia species complex (Hymenoptera: Pteromalidae)
Stefan Friedrich Wirth
A high-coverage genome from a 200,000-year-old Denisovan
AND
A global map for introgressed structural variation and selection in humans
Siddharth Singh
Dissecting Gene Regulatory Networks Governing Human Cortical Cell Fate
Manuel Lessi
preLists in the developmental biology category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
BSDB/GenSoc Spring Meeting 2024
A list of preprints highlighted at the British Society for Developmental Biology and Genetics Society joint Spring meeting 2024 at Warwick, UK.
| List by | Joyce Yu, Katherine Brown |
GfE/ DSDB meeting 2024
This preList highlights the preprints discussed at the 2024 joint German and Dutch developmental biology societies meeting that took place in March 2024 in Osnabrück, Germany.
| List by | Joyce Yu |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
2nd Conference of the Visegrád Group Society for Developmental Biology
Preprints from the 2nd Conference of the Visegrád Group Society for Developmental Biology (2-5 September, 2021, Szeged, Hungary)
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Society for Developmental Biology 79th Annual Meeting
Preprints at SDB 2020
| List by | Irepan Salvador-Martinez, Martin Estermann |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EDBC Alicante 2019
Preprints presented at the European Developmental Biology Congress (EDBC) in Alicante, October 23-26 2019.
| List by | Sergio Menchero et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |
Pattern formation during development
The aim of this preList is to integrate results about the mechanisms that govern patterning during development, from genes implicated in the processes to theoritical models of pattern formation in nature.
| List by | Alexa Sadier |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |
Also in the evolutionary biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
EMBO | EMBL Symposium: The organism and its environment
This preList contains preprints discussed during the 'EMBO | EMBL Symposium: The organism and its environment', organised at EMBL Heidelberg, Germany (May 2023).
| List by | Girish Kale |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
COVID-19 / SARS-CoV-2 preprints
List of important preprints dealing with the ongoing coronavirus outbreak. See http://covidpreprints.com for additional resources and timeline, and https://connect.biorxiv.org/relate/content/181 for full list of bioRxiv and medRxiv preprints on this topic
| List by | Dey Lab, Zhang-He Goh |
1
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Pattern formation during development
The aim of this preList is to integrate results about the mechanisms that govern patterning during development, from genes implicated in the processes to theoritical models of pattern formation in nature.
| List by | Alexa Sadier |











 (4 votes)
(4 votes)