Embryo geometry drives formation of robust signaling gradients through receptor localization
Posted on: 3 January 2019 , updated on: 21 October 2019
Preprint posted on 9 December 2018
Article now published in Nature Communications at https://www.nature.com/articles/s41467-019-12533-7
Mind the gap: epiblast geometry at its extraembryonic boundary constrains BMP localization and ensures robust gradient formation.
Selected by Paul Gerald L. Sanchez and Stefano VianelloCategories: developmental biology
Background
The early mouse embryo is patterned by a rich landscape of overlapping gradients of signalling activities, which allow cells to leverage the type, the timing and the strength of these signals as a read-out of position, thus ensuring correct allocation of fates. In mouse (as in human), an important contribution to these signalling cues and to their organisation comes not from the embryo, but from extraembryonic tissues. Bone Morphogenic Protein 4 (BMP4), secreted by the Extraembryonic Ectoderm (ExE, cfr. Figure A), is one of such cues. Accordingly, in the E6.0 mouse embryo a sharp band of BMP expression marks the entire boundary where the ExE meets the embryonic epiblast (EPI). EPI cells detect these molecules through the BMP Receptor 1a (BMPR1a, also known as Alk3). Consequently, the detection of BMP4 provides a read-out of relative position, and specifically of closeness to the ExE to the otherwise equivalent cells of the early EPI. Active BMP signalling restricted to the proximal boundary of the EPI contributes to important developmental events such as Primordial Germ Cell specification, and extraembryonic and posterior mesoderm development.
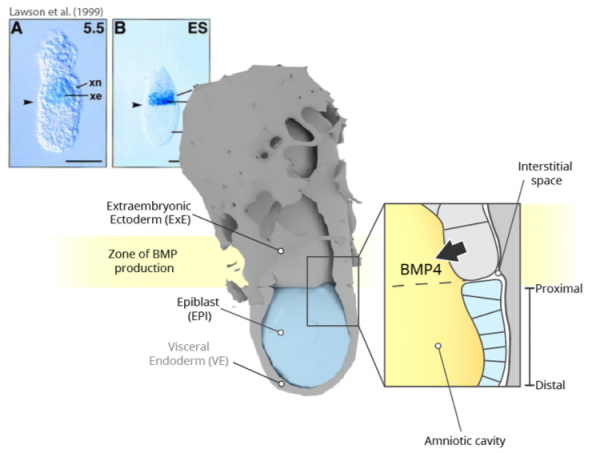
On the BMP zone of production and the embryonic-extraembryonic boundary
While the textbook picture described above makes intuitively sense (patterning of the proximal EPI by proximally-restricted BMP activity, in turn explained by a proximally-located extraembryonic source), it clashes with the – often overlooked – geometrical reality of the mouse embryo. Indeed, how can the signalling activity of BMP4 molecules be restricted to the proximal EPI given that BMPs are likely secreted into the amniotic cavity, spanning the entire length of the embryo?
Key findings
The preprint highlights the importance of restricted receptor localization, and consequently the embryo’s geometry, in the establishment of a robust signalling gradient of a morphogen. Using complementary in silico, in vitro, and in vivo approaches, the authors investigate the extracellular dynamics of BMP through the interstitial fluid, from its source to its destination. Simulations show that regardless of where BMP molecules are exactly secreted within the embryo, they accumulate within the amniotic cavity: BMP molecules have access to the entire length of the embryo as they are produced. Crucially, indiscriminate signalling activity across the entire EPI is prevented by the compartmentalisation of BMP receptors to the opposite (basolateral) side, making cells unresponsive to apically-presented ligands.
A very restricted subset of EPI cells (those at the ExE-EPI boundary) does however respond to BMP in vivo. How does this happen? A major premise of the study is the identification of a channel, at this very boundary and at the embryo’s presumptive posterior, allowing communication between the amniotic cavity and the interstitial fluid on the opposite side. By diffusing across this only gap, BMP can infiltrate the basolateral interstitial space and bind to EPI receptors. Simulations show that this configuration is sufficient to establish a signalling gradient from the edge of the EPI inwards: as molecules enter the confined space of the basolateral interstitium, they will bind the closest available receptor, with lower and lower chances of reaching more distal cells (cfr. Figure B).
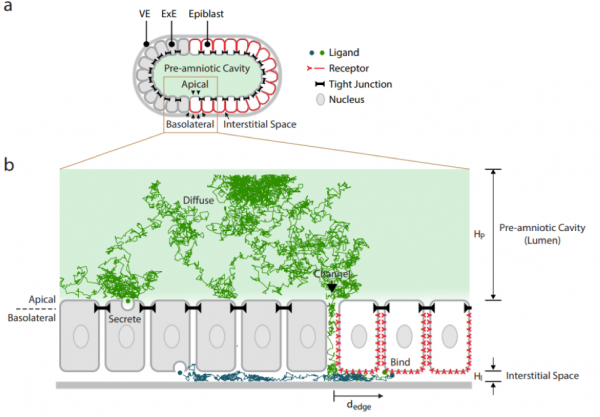
Simulation of BMP extracellular movement in the mouse embryo
Predictions of the model were tested in hESC monolayers (as these too have basolaterally-localized BMP receptors), with consistent validation in the mouse embryo. Pharmacological disruption of tight junctions between responding cells broadened the domain of induced downstream signalling, and mislocalization of BMP receptors on the apical side resulted in ectopic BMP activity. The importance of geometry was also highlighted in experiments using embryos without ExE and/or visceral endoderm (VE in Figure A), bathed in BMP. Taking into account both receptor localization and geometry, the preprint identifies the distance from the edge of the EPI – rather than from the signalling source – as the major determinant of EPI patterning in the mouse embryo. It also highlights the amniotic cavity as a buffer of morphogen levels, ensuring robustness of patterning across variable developmental conditions.
Significance
The authors underscore a crucial yet under-appreciated point of developmental patterning: given that signalling receptors are not homogeneously distributed along cellular membranes (at least for BMP signalling), their orientation relative to different embryonic compartments becomes critical to their ability to respond to surrounding cues. What matters now is not anymore the proximity of signalling sources, but the accessibility to the signals. Another significant observation is the identification of reservoirs of signalling determinants (here the amniotic cavity), acting as buffers of signalling fluctuations during embryonic development. More broadly, the paper joins an increasing body of literature highlighting mechanics and geometry – and not just biochemistry – as instructive cues of development.
From an experimental point of view, we want to highlight the incredible ability of the authors to achieve control over receptor localisation. Indeed, such a strategy could be leveraged in other systems where density-dependent differential ligand accessibility to receptors has been demonstrated to underlie differentiation and fate positioning [cfr. micropatterned colonies, Etoc et al, 2016]. We also appreciate the new data about in vivo BMPR1a localisation, as well as the use of microfluidics to discriminate between alternative coordinate systems (distance from source versus distance from edge) and to correlate this to cell fate.
Recently, a preprint by Recho, Hallou, and Hannezo described how a gradient of a single morphogen could generate advective interstitial fluid flow, refining the gradient profile and eliciting a concentration-sensitive mechano-chemical tissue response (Recho et al, 2018). It would be interesting to investigate if this is the case for BMP signalling in the pre-gastrulation mouse embryo.
Open questions
- Is the channel between the amniotic cavity and the interstitial space, marking the ExE-EPI boundary, only at the proximal presumptive posterior side? Is there a similar channel at the presumptive anterior side?
- How does the model fit with the fact that later during gastrulation the interstitial space becomes invaded by mesodermal cells? Is the gradient maintained?
- Is BMPR1A also graded from the edge of EPI inwards? What would happen if receptors were selectively removed from the first section of cells on the edge? Do you expect a distal shift of the signalling gradient?
preLighter Diana Pinheiro has also highlighted this preprint – check out her highlight here
Further reading
To read about classical model of EPI patterning by BMP:
- Stower, Matthew J., and Shankar Srinivas. “The Head’s Tale: Anterior-Posterior Axis Formation in the Mouse Embryo.” Current topics in developmental biology. Vol. 128. Academic Press, 2018. 365-390. https://doi.org/10.1016/bs.ctdb.2017.11.003.
- Lawson, Kirstie A., et al. “Bmp4 is required for the generation of primordial germ cells in the mouse embryo.” Genes & development 13.4 (1999): 424-436. https://doi.org/10.1101/gad.13.4.424.
- Winnier, Glenn, et al. “Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse.” Genes & development 9.17 (1995): 2105-2116. https://doi.org/10.1101/gad.9.17.2105.
- Waldrip, W. Ross, et al. “Smad2 signaling in extraembryonic tissues determines anterior-posterior polarity of the early mouse embryo.” Cell 92.6 (1998): 797-808. https://doi.org/10.1016/S0092-8674(00)81407-5.
For more details about the architecture of the early mouse embryo:
- Christodoulou, Neophytos, et al. “Sequential formation and resolution of multiple rosettes drive embryo remodelling after implantation.” Nature cell biology 20.11 (2018): 1278. https://doi.org/10.1038/s41556-018-0211-3.
Previous studies on pSmad gradients in micropatterned human and mouse systems:
- Morgani, Sophie M., et al. “Micropattern differentiation of mouse pluripotent stem cells recapitulates embryo regionalized cell fate patterning.” Elife 7 (2018): e32839. https://doi.org/10.7554/eLife.32839.001.
- Etoc, Fred, et al. “A balance between secreted inhibitors and edge sensing controls gastruloid self-organization.” Developmental cell 39.3 (2016): 302-315. https://doi.org/10.1016/j.devcel.2016.09.016.
- Tewary, Mukul, et al. “A stepwise model of Reaction-Diffusion and Positional-Information governs self-organized human peri-gastrulation-like patterning.” Development (2017): dev-149658. https://doi.org/10.1242/dev.149658.
For other examples of the effect of geometry on early mouse development:
- Blin, Guillaume, et al. “Geometrical confinement controls the asymmetric patterning of brachyury in cultures of pluripotent cells.” Development 145.18 (2018): dev166025. https://doi.org/10.1242/dev.166025.
- Hiramatsu, Ryuji, et al. “External mechanical cues trigger the establishment of the anterior-posterior axis in early mouse embryos.” Developmental cell 27.2 (2013): 131-144. https://doi.org/10.1016/j.devcel.2013.09.026.
Preprint on mechano-chemical patterning by morphogens:
- Recho, Pierre, Adrien Hallou, and Edouard Hannezo. “Theory of mechano-chemical patterning in biphasic biological tissues.” arXiv preprint arXiv:1811.12242(2018). https://arxiv.org/abs/1811.12242.
doi: https://doi.org/10.1242/prelights.6820
Read preprintHave your say
Sign up to customise the site to your preferences and to receive alerts
Register hereAlso in the developmental biology category:
Cooperation between cortical and cytoplasmic forces shapes planar 4-cell stage embryos
Corentin Mollier, Shivani Dharmadhikari
A drought stress-induced MYB transcription factor regulates pavement cell shape in leaves of European aspen (Populus tremula)
Jeny Jose
Cross Sectional and Longitudinal Imaging Reveals Spatiotemporal Divergence in Morphogenesis and Cell Lineage Specification between in-vivo and in-vitro Mouse Embryo during Pre- and Peri-implantation
Heather Pollington
preLists in the developmental biology category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
BSDB/GenSoc Spring Meeting 2024
A list of preprints highlighted at the British Society for Developmental Biology and Genetics Society joint Spring meeting 2024 at Warwick, UK.
| List by | Joyce Yu, Katherine Brown |
GfE/ DSDB meeting 2024
This preList highlights the preprints discussed at the 2024 joint German and Dutch developmental biology societies meeting that took place in March 2024 in Osnabrück, Germany.
| List by | Joyce Yu |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
2nd Conference of the Visegrád Group Society for Developmental Biology
Preprints from the 2nd Conference of the Visegrád Group Society for Developmental Biology (2-5 September, 2021, Szeged, Hungary)
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Society for Developmental Biology 79th Annual Meeting
Preprints at SDB 2020
| List by | Irepan Salvador-Martinez, Martin Estermann |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EDBC Alicante 2019
Preprints presented at the European Developmental Biology Congress (EDBC) in Alicante, October 23-26 2019.
| List by | Sergio Menchero et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |
Pattern formation during development
The aim of this preList is to integrate results about the mechanisms that govern patterning during development, from genes implicated in the processes to theoritical models of pattern formation in nature.
| List by | Alexa Sadier |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |











 (5 votes)
(5 votes)
7 years
Eric Siggia
The reference to the paper of Etoc et al Dev Cell 2016 “asymmetric receptor localisation has been postulated to underlie patterning” needs to be corrected. As the title (“..Edge Sensing..”) and abstract make clear, our paper shows experimentally that for micropatterned hESC colonies, receptors to both BMP and Activin are only accessible to apical applied ligands at the boundaries of the colonies. This phenomena is density dependent. This is shown by visualizing the receptors and also culturing the cells on filters so their apical vs basal responses can be quantified. The discussion very explicitly remarks that the mouse embryo should operate in the same fashion since ligands released into the amniotic cavity would rapidly be accessible everywhere.
This is in no way meant to detract from the very important paper from the Ramanathan lab, highlighted here.