Gene complementation analysis suggests that dodder plants (Cuscuta spp.) do not depend on the host FT protein for flowering
Posted on: 31 January 2023
Preprint posted on 19 December 2022
Host-independent flowering of Cuscuta spp. reignites the search for a ‘Florigen’
Selected by Gwendolyn K. Kirschner, Marc SomssichCategories: developmental biology, plant biology
Background:
The search for a mysterious, leaf-derived substance that may induce flowering in the shoot meristem of plants was a hot topic in the 20th century (Somssich, 2020). Already in 1863 did the German botanist Julius Sachs speculate that such a substance must exist, and in the 1930s, Mikhail K. Chailakhyan conducted a series of elegant experiments to show that it is indeed the leaves that perceive day-length via light, and that it is sufficient to expose the leaves to a specific light regime to induce flowering in a differently treated shoot (Sachs J., 1865; Chailakhyan, 1968). Chailakhyan suggested that it could be a phytohormone that acts as messenger, and provisionally named it ‘florigen’ (blossom-former). In Lysenkoist Russia, this hormonal, rather than Marxist theory of plant development was unacceptable. Science had to be tailored to fit the communist party line, which dictated that life was controlled through external forces, not internal factors, such as hormones. Thus, his work cost Chailakhyan his PhD, job and career – but the quest to identify florigen continued to intrigue plant scientists until the early 2000s (Somssich, 2020). It was only in 2007, that four independent studies identified the gene product of the FLOWERING LOCUS T (FT) to be the mysterious florigen (Corbesier et al., 2007; Jaeger and Wigge, 2007; Mathieu et al., 2007; Tamaki et al., 2007).
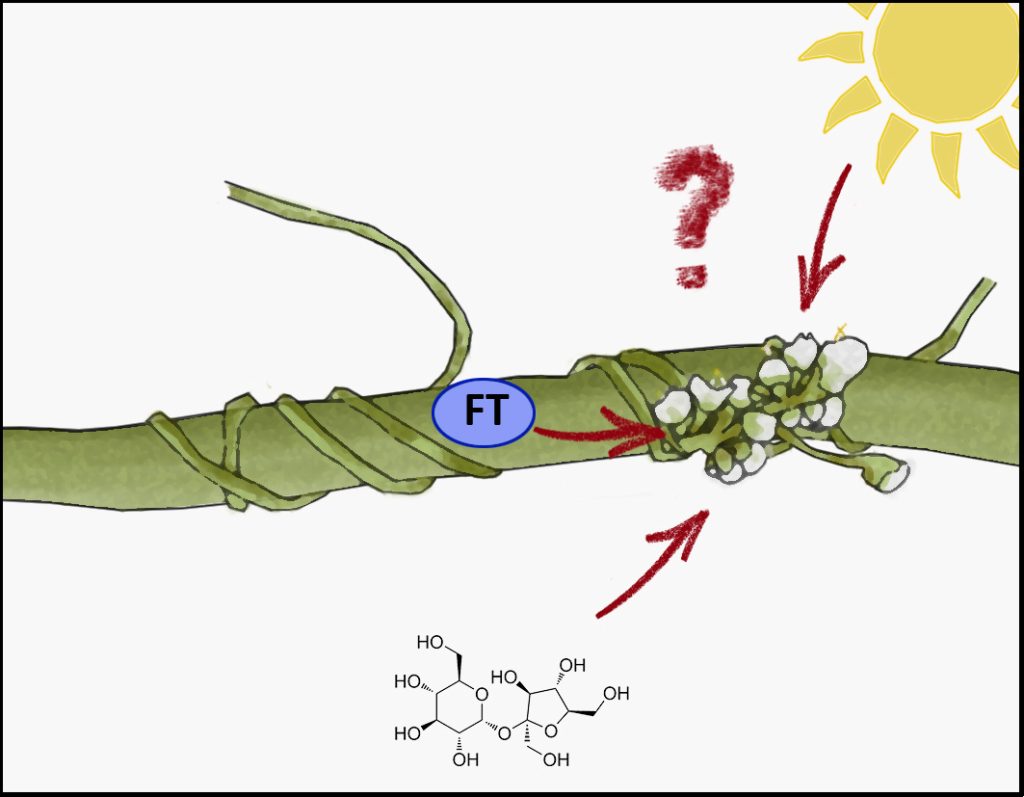
Curiously, it now appears that we once again find ourselves hunting for a mobile, flower-inducing signal – and this time it may not be FT!
Cuscuta ssp. (dodder) is a leaf- and rootless parasitic plant with little photosynthetic activity that depends entirely on a host plant to supply it with water, nutrients, and carbohydrates to complete its life cycle. Following germination, the Cuscuta seedling senses a suitable host in its periphery via excreted plant volatiles that act as chemo-attractants (Kaiser et al., 2015). Among the susceptible hosts are many crop plants, such as potato, tomato, sugar beet and melons (Mishra and Kogan, 2009). After locating the host, the Cuscuta stems wind around the host shoots and develop a specialized organ at the contact site, the haustorium, to penetrate the host tissue and establish a connection to its xylem (Lee, 2007). Importantly, this interface does not just allow the parasite to drain nutrients, solutes and carbohydrates from the host, but also the exchange of macromolecules like RNA and proteins (Kaiser et al., 2015). Furthermore, the parasite also synchronizes its lifecycle with that of the host, including its flowering time. It therefore seemed reasonable to assume that the mobile FT is among the proteins transferred to the parasite to induce flowering at the same time as the host, and indeed, this is what a recent study by Shen et al. (2020) suggested.
Shen et al., (2020) proposed that Cuscuta australis uses FT from its host plant Arabidopsis to synchronize its own flowering time with that of the host (Shen et al., 2020). For the parasite, this would enable it to make use of the host’s whole life cycle for feeding, while not jeopardizing its own propagation by ending its life cycle too late. The authors provided different lines of evidence for this: the Cuscuta genome lost many genes related to flowering time regulation in Arabidopsis. C. australis FT expression was not detected in stems before, while, or after the transition to the reproductive stage. Short day conditions suppressed the endogenous expression of AtFT, and misexpression of CaFT in Arabidopsis did not induce flowering under these conditions either, suggesting that CaFT is inactive. The authors could also confirm the presence of AtFT in C. australis, interaction between AtFT and the C. australis homolog of the essential FT co-factor FD, as well as the activation of flowering related genes. Thus, it indeed appeared that host FT was transferred to the parasite and was functional there. However, the author’s conclusion, that Cuscuta ‘eavesdrops on the host’s FT signal’, was recently queried, when Bernal-Galeano et al. (2022) established an in vitro system for the growth of Cuscuta campestris, in which the parasite grows to flowering stage despite the use of a paper spindle from a cotton swab as “host” (Bernal-Galeano et al., 2022).
In the new preprint discussed here, Mäckelmann and Känel (equal contribution) et al. (2022) investigate this phenomenon further and find that C. campestris has its own FT proteins, which are sufficient to induce flowering of the parasite – even in the absence of a host FT.
Key findings:
The authors used the tobacco (Nicothiana tabacum)-C. campestris host-parasite-system for their work since tobacco is day-length neutral and double mutants of the two floral inducers (Ntft4/Ntft5) do not flower at all. Using these plants, the authors found that C. campestris flowered after ~ 40 days – both in wild type and FT-less Ntft4/Ntft5 double mutant plants.
Analysing genomic and transcriptomic data, the authors then identified hundreds of flowering time-associated protein-coding genes, and observed that only two had Cuscuta-specific deletions, while other gene losses or divergence from the Arabidopsis coding sequences where shared with other eudicots, and therefore most likely inconsequential for flowering. C. campestris has two FT homologues, CcFT1 and CcFT2, and the amino acid sequence of CcFT1 was identical to the C. australis FT homologue. They furthermore identified two and one FD-like genes in C. campestris and C. australis, respectively. qRT-PCR and RNAseq analysis showed expression of C. campestris and C. australis FD-likes in all tissues examined, while expression of their respective FT homologs was specific to haustoria.
Using BiFluorescence Complementation assays, the authors then went on to show that CcFT1 and CcFT2 can interact with the FDs from both C. campestris and tobacco, and that CcFD-like1 can interact with NtFT5, suggesting a conserved interaction across plant species. Given that the amino acid sequence of CcFT1 is identical to CaFT, it can be assumed that CaFT shares these interactions.
Finally, the authors tested the functionality of CcFT in tobacco plants by overexpressing CcFT1 in the Ntft4/Ntft5 double mutant plants and found that CcFT1 was able to restore the flowering capacity of the mutant to wild type level.
Conclusions:
The hypothesis that Cuscuta lost its ability to flower without a flowering host, is partially based on the loss of genes in Cuscuta that are involved in flowering in Arabidopsis. However, this only holds true if we consider the flowering control of Arabidopsis a universal concept. But in fact, Mäckelmann and Känel et al. (2022) could show that gene losses and divergence also occur among other flowering plants when compared to Arabidopsis, so gene loss or divergence as such cannot be used as evidence.
In contrast to Shen et al. (2020), Mäckelmann and Känel et al. (2022) could detect expression of Cuscuta FT in both species, C. australis and C. campestris, because they included the haustorial tissue. Haustoria are the interface between the parasitic plant and the host and are therefore crucial for sensing the physiological status of the hosts. Instead of the transfer of host-derived FT protein, the endogenous production of FT could be triggered in the Cuscuta haustoria by other host-derived metabolites, such as sucrose. Sucrose, coupled to its production in photosynthesis and thereby an indirect read-out of the photoperiod and flowering induction of the host, was shown before to induce the expression of FT (King et al., 2008).
In conclusion, transfer of the host FT to the parasite does not appear to be required for the parasite to flower. Nonetheless, it is true that the parasite synchronizes its flowering time to the host timing, and therefore there must be a host-derived signal to provide a cue for flowering. Thus, we once again find ourselves searching for a mobile, flower-inducing signal. A blossom-former, another florigen. This substance could be another protein, a metabolite, and possibly even a phytohormone – as suggested by Chailakhyan in 1936.
Why we think this preprint is important:
The study underlines how small deviations in an experimental setup (such as working with the different plant species C. australis and C. campestris from the same genus), or different pre-assumptions can lead to contrasting conclusions. Furthermore, it reinforces the fact that we should always consider different plant models for universal conclusions about mechanisms and pathways. At the same time, it illustrates how each study complements previous work, and how, piece by piece and publication by publication, our understanding of a subject deepens.
Future directions and question for the authors:
It was shown before that C. australis only flowers when the host plant is able to flower, and that the flowering times are highly synchronized (Shen et al., 2020). In case of C. campestris, however, flowering is possible even when grown on the Ntft4/Ntft5 double mutant. Is this due to the experimental conditions, for example the light regime, or determined by the different Cuscuta species (i.e., did the authors try to reproduce the results of Shen et al. (2020) in their lab)?
An important experiment in this regard would be the knock-out of the haustorial expressed CaFT in C. australis, to see if this mutant would still flower. If a Caft mutant still flowers, and does so in sync with the host, this would be a very good indication that it is indeed the host FT that induces flowering in C. australis. Such an experiment could be conducted by decreasing CaFT expression by RNAi or CRISPR/Cas9 with the available Cuscuta transformation protocol, which targets the adhesive discs of the haustorium, the relevant tissue in this context (Lachner et al., 2020).
If it is indeed due to the different Cuscuta species, it would be very interesting to find out why C. australis has developed this additional mechanism of adaptation. Further to this, it could be interesting to look into the role of CcFT2, since this homolog is lost in C. australis. Does misexpression of CcFT2 in C. australis change its flowering time?
References:
Bernal-Galeano, V., Beard, K. and Westwood, J.H. (2022) An artificial host system enables the obligate parasite Cuscuta campestris to grow and reproduce in vitro. Plant Physiol., 189, 687–702.
Chailakhyan, M.K. (1968) Internal Factors of Plant Flowering. Annu. Rev. Plant Physiol., 19, 1–37.
Corbesier, L., Vincent, C., Jang, S., et al. (2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science (80-. )., 316, 1030–1033.
Jaeger, K.E. and Wigge, P.A. (2007) FT Protein Acts as a Long-Range Signal in Arabidopsis. Curr. Biol., 17, 1050–1054.
Kaiser, B., Vogg, G., Fürst, U.B. and Albert, M. (2015) Parasitic plants of the genus Cuscuta and their interaction with susceptible and resistant host plants. Front. Plant Sci., 6, 1–9.
King, R.W., Hisamatsu, T., Goldschmidt, E.E. and Blundell, C. (2008) The nature of floral signals in Arabidopsis. I. Photosynthesis and a far-red photoresponse independently regulate flowering by increasing expression of FLOWERING LOCUS T (FT). J. Exp. Bot., 59, 3811–3820.
Lachner, L.A.M., Galstyan, L. and Krause, K. (2020) A highly efficient protocol for transforming Cuscuta reflexa based on artificially induced infection sites. Plant Direct, 4, 1–11.
Lee, K.B. (2007) Structure and development of the upper haustorium in the parasitic flowering plant Cuscuta japonica (Convolvulaceae). Am. J. Bot., 94, 737–745.
Mathieu, J., Warthmann, N., Küttner, F. and Schmid, M. (2007) Export of FT Protein from Phloem Companion Cells Is Sufficient for Floral Induction in Arabidopsis. Curr. Biol., 17, 1055–1060.
Mishra, J.S. and Kogan, M. (2009) Biology and Management of Cuscuta species. Indian J. Weed Sci., 41, 1–11.
Sachs J. (1865) Untersuchungen über die allgemeinsten Lebensbedingungen der Pflanzen und die Functionen ihrer Organe. In Handbuch der Experimental-Physiologie der Pflanzen. Leipzig: Wilhelm Engelmann.
Shen, G., Liu, N., Zhang, J., Xu, Y., Baldwin, I.T. and Wu, J. (2020) Cuscuta australis (dodder) parasite eavesdrops on the host plants’ FT signals to flower. Proc. Natl. Acad. Sci. U. S. A., 117, 23125–23130.
Somssich, M. (2020) A Short History of Vernalization. Zenodo.
Tamaki, S., Matsuo, S., Wong, H.L., Yokoi, S. and Shimamoto, K. (2007) Hd3a Protein Is a Mobile Flowering Signal in Rice. Science (80-. )., 316, 1033–1036.
doi: https://doi.org/10.1242/prelights.33586
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the developmental biology category:
Tissue mechanics and systemic signaling safeguard epithelial tissue against spindle misorientation
Ruoheng Li
Human pluripotent stem cell-derived macrophages modify development of human kidney organoids
Theodora Stougiannou
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
Also in the plant biology category:
Actin Counters Geometry to Guide Plant Cell Division
Jeny Jose
The nucleus follows an internal cellular scale during polarized root hair cell development
Jeny Jose
Conservation and divergence of regulatory architecture in nitrate-responsive plant gene circuits
Jeny Jose
preLists in the developmental biology category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
BSDB/GenSoc Spring Meeting 2024
A list of preprints highlighted at the British Society for Developmental Biology and Genetics Society joint Spring meeting 2024 at Warwick, UK.
| List by | Joyce Yu, Katherine Brown |
GfE/ DSDB meeting 2024
This preList highlights the preprints discussed at the 2024 joint German and Dutch developmental biology societies meeting that took place in March 2024 in Osnabrück, Germany.
| List by | Joyce Yu |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
2nd Conference of the Visegrád Group Society for Developmental Biology
Preprints from the 2nd Conference of the Visegrád Group Society for Developmental Biology (2-5 September, 2021, Szeged, Hungary)
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Society for Developmental Biology 79th Annual Meeting
Preprints at SDB 2020
| List by | Irepan Salvador-Martinez, Martin Estermann |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EDBC Alicante 2019
Preprints presented at the European Developmental Biology Congress (EDBC) in Alicante, October 23-26 2019.
| List by | Sergio Menchero et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |
Pattern formation during development
The aim of this preList is to integrate results about the mechanisms that govern patterning during development, from genes implicated in the processes to theoritical models of pattern formation in nature.
| List by | Alexa Sadier |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |
Also in the plant biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |











 (No Ratings Yet)
(No Ratings Yet)