Presence of midline cilia supersedes the expression of Lefty1 in forming the midline barrier during the establishment of left-right asymmetry
Posted on: 8 June 2018
Preprint posted on 17 May 2018
Keep Left! A requirement for cilia to establish and maintain the midline barrier during left-right symmetry breaking.
Selected by Hannah BrunsdonCategories: developmental biology
Background
The breakage of left-right symmetry during development is a process that has both fascinated and frustrated developmental biologists for decades. During early development, cells in an initially symmetrical embryo must acquire positional identities along the left/right (L-R) axis to ensure that organs later develop in the correct position, and then if required, go on to develop asymmetrically themselves.
In vertebrates, the initial breakage in symmetry is thought to originate from the beating of polarised cilia, which produce a leftward flow of extraembryonic fluid within the left-right organiser region (the node in mammals). Another type of cilia sense this flow, and activate the left-determinant gene Nodal in the left lateral plate mesoderm (LPM) only. Importantly, expression of the Nodal inhibitor Lefty1 forms a barrier along the midline to prevent activation of Nodal on the right-hand side. Nodal then propagates an asymmetric signalling cascade to correctly pattern the developing embryo along the L-R axis[1].
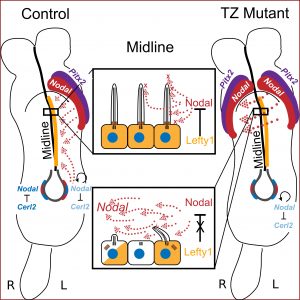
Graphical abstract, kindly provided by the Weatherbee lab: In a wild-type embryo, Nodal and Pitx2 are restricted to the left-hand side due to the midline barrier, which is established and maintained by midline cilia and Lefty1-mediated Nodal inhibition. However, in transition zone (TZ) mutants, midline cilia are greatly reduced in number and Lefty1 expression is not sufficient to inhibit Nodal. Consequently, Nodal diffuses across the midline barrier and the its signalling cascade is activated on both sides. Ultimately, this leads to numerous laterality defects later in development.
Many questions how the Nodal signalling cascade is initiated, maintained and transduced remain to be answered. Even the exact roles and importance of cilia to the initial breakage of L-R patterning are somewhat controversial, as the iv mouse mutant for example has immotile cilia, but 25% of embryos develop normally[1]. Discrepancies like these between theories that explain phenotypes of some mutants very well, but not of others, suggest that there is still much to understand about the establishment and maintenance of L-R asymmetry.
In this preprint, Shylo and colleagues investigated L-R patterning in three different mouse ciliary mutants. At first, their phenotypes seemed contradictory to each other, and also differed from current models. After investigating these inconsistencies further, the authors unexpectedly discovered a novel requirement for midline cilia to establish and maintain the midline barrier, independent of Lefty1 expression.
Key findings
- The authors first studied mouse embryos containing a null mutation in Tmem107 (Tmem107null), a gene which encodes a component of the transition zone of primary cilia. Tmem107null embryos developed L-R asymmetry defects such as randomised heart looping, stomach positioning and left pulmonary isomerism. Earlier in development, the nodes of Tmem107null embryos were found to contain fewer cilia, although those present appeared normal. In terms of gene expression, in situ hybridisation showed that Nodal and Pitx2 were expressed bilaterally in the LPM of Tmem107null embryos, in contrast to wild type controls, where Nodal and its target Pitx2 were confined to the left-hand side.
- The formation of the midline barrier, and Lefty1 expression, is thought to be dependent on Sonic Hedgehog (Shh) signalling[2]. In Tmem107null embryos, the Shh targets FoxA2 and Ptch1 were reduced at the midline compared to controls, and Lefty1 was absent. From this the authors concluded that without functional Tmem107, Shh signalling is not properly transduced, leading to loss of Lefty1 and a defective midline barrier.
- The authors next investigated another ciliary transition zone mutant Mks1 (Mks1krc), known to have the same laterality defects as Tmem107null embryos[3]. Similar to Tmem107null embryos, Nodal and Pitx2 were bilaterally expressed, and Shh targets absent. Surprisingly, Lefty1 expression was present in the midline of Mks1krc. This contradicts previous work suggesting that firstly Lefty1 in the midline barrier is Shh-dependent, and secondly that Lefty1 is sufficient for establishing the midline barrier.
- Further questions arose after authors investigated the hypomorphic Tmem107 mutant (Tmem107schlei) mouse line, which despite containing fewer and abnormally-shaped nodal cilia, exhibit no laterality defects[4]. Interestingly however, a population of cilia were spotted in the midlines of wild-type and Tmem107schlei embryos, which were absent in the midlines of both Tmem107null and Mks1krc embryos.
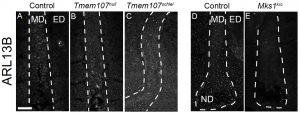
From Figure 8 – used with permission from the authors. Midline cilia are absent in transition zone ciliary mutants that later develop laterality defects: Confocal Z-stacks show the node and/or midlines of Tmem107schlei, Tmem107null and Mks1krc embryos, after immunostaining with an antibody against the cilia marker ARL13B. Cilia are almost absent in Tmemnull and Mks1krc midlines, but can be observed in Tmem107schlei midlines and wild-type controls.
Why I chose this paper
The subject of how cells acquire and process positional information has always been fascinating to me, and the heated debates about cilia and L-R asymmetry were used as literature review exercises multiple times during my university days. Although this paper does not go into the function of the midline cilia, it provides another piece of the L-R asymmetry puzzle, which might eventually show us how L-R patterning is defined, maintained, and then propagated in later development.
As a general reason, I think there is a temptation to put experimental findings to one side when they contradict current theories, or to cherry pick data until they fit, instead of exploring confusing results further. In this preprint, Shylo and colleagues show a series of simple yet directed experiments to investigate unexpected results, which lead them to discover something novel which will hopefully be very interesting for follow-up research.
Open questions
- For me, the biggest question from this work is what the function of midline cilia might be– are they involved in Shh signalling or sensing to maintain the midline barrier? I wonder whether midline cilia are present in other ciliary mutants which have unexpected laterality phenotypes.
- Connected to the above point, it might be interesting to know if the few cilia that are present in the midline or nodes of Tmem107null and Mks1krc mutants are phenotypically normal. Even though they might be positive for cilia markers, do the nodal cilia still beat, or are any midline cilia present the same length and shape as in wild type?
- As well as Shh signalling, other developmental pathways such as Wnt and FGF signalling are important for nodal cilia function in L-R asymmetry– I wonder if any of these developmental signalling pathway components are also disrupted in Tmem107null, Tmem107schlei and Mks1krc mutants, and if they also contribute to midline establishment and function.
References
- Schweikert, A., et al. (2018). Vertebrate Left-Right Asymmetry: What can Nodal Cascade Gene Expression Patterns Tell Us? J. Cardiovasc. Dev. Dis 5, 1.
- Tsiairis, C.D. and McMahon, A.P. (2009) An Hh-dependent pathway in lateral plate mesoderm enables the generation of left/right asymmetry. Curr. Biol. CB, 19,1912-1917.
- Weatherbee, S.D., Niswander, L.A. and Anderson, K.V. (2009) A mouse model for Meckel syndrome reveals Mks1 is required for ciliogenesis and Hedgehog signalling. Human molecular genetics. 18, 4565-4575.
- Christopher, K.J., Wang, B., Kong, Y, et al. (2012). Forward genetics uncovers Transmembrane protein 107 as a novel factor required for ciliogenesis and Sonic hedgehog signalin. Dev. Bio. 368, 382-392
Sign up to customise the site to your preferences and to receive alerts
Register hereAlso in the developmental biology category:
Cooperation between cortical and cytoplasmic forces shapes planar 4-cell stage embryos
Corentin Mollier, Shivani Dharmadhikari
A drought stress-induced MYB transcription factor regulates pavement cell shape in leaves of European aspen (Populus tremula)
Jeny Jose
Cross Sectional and Longitudinal Imaging Reveals Spatiotemporal Divergence in Morphogenesis and Cell Lineage Specification between in-vivo and in-vitro Mouse Embryo during Pre- and Peri-implantation
Heather Pollington
preLists in the developmental biology category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
BSDB/GenSoc Spring Meeting 2024
A list of preprints highlighted at the British Society for Developmental Biology and Genetics Society joint Spring meeting 2024 at Warwick, UK.
| List by | Joyce Yu, Katherine Brown |
GfE/ DSDB meeting 2024
This preList highlights the preprints discussed at the 2024 joint German and Dutch developmental biology societies meeting that took place in March 2024 in Osnabrück, Germany.
| List by | Joyce Yu |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
2nd Conference of the Visegrád Group Society for Developmental Biology
Preprints from the 2nd Conference of the Visegrád Group Society for Developmental Biology (2-5 September, 2021, Szeged, Hungary)
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Society for Developmental Biology 79th Annual Meeting
Preprints at SDB 2020
| List by | Irepan Salvador-Martinez, Martin Estermann |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EDBC Alicante 2019
Preprints presented at the European Developmental Biology Congress (EDBC) in Alicante, October 23-26 2019.
| List by | Sergio Menchero et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |
Pattern formation during development
The aim of this preList is to integrate results about the mechanisms that govern patterning during development, from genes implicated in the processes to theoritical models of pattern formation in nature.
| List by | Alexa Sadier |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |











 (No Ratings Yet)
(No Ratings Yet)