Single-molecule live cell imaging of the Smc5/6 DNA repair complex
Posted on: 26 June 2020
Preprint posted on 20 June 2020
Article now published in eLife at http://dx.doi.org/10.7554/eLife.68579
Smc5/6 and the chromatin: Shedding light on this complex relationship using sptPALM
Selected by Jennifer Ann BlackCategories: molecular biology
Background:
Our chromosomes (linear DNA molecules) are arranged and folded into a small confined space; the nucleus. How chromosomes fold, their placement in the nucleus and their ability to interact with each other (collectively their ‘higher-order’ structure) all contribute to modulating gene expression. In Eukaryotes, several complexes act in nuclear chromosome organisation including four Structural Maintenance of Chromosomes (SMC) complexes; the dosage dependent complex, cohesin, condensin and the Smc5/6 complex. The Smc5/6 complex, whose diverse roles include associating with double strand breaks (DSB) to assist homologous recombination (HR) repair, telomeres, the ribosomal DNA (rDNA) array and replication forks, is composed of Smc5 and Smc6 (1). Individually, both proteins look like long ‘arms’ meeting at a circular doughnut shaped region (‘hinge’) at one end (thought to interact with DNA) and a globular region at the other end which contains ATPase activity; little is known about the role of ATPase activity in the Smc5/6 complex. At the globular ATPase region, a sub-complex can interact to support Smc5/6 functionality: Kleisin (Nse4) and two KITE proteins (Nse1 and Nse3). Other Nse proteins also interact, including Nse2 (interacts with Smc5’s arm) and Nse5 and Nse6 (in yeast) (2). Despite Smc5/6 having important chromatin-based roles, much of the dynamics of its interaction with the chromatin remains unknown. Here, the authors used sophisticated microscopy and Smc5/6 mutants to understand these reaction dynamics.
Key Findings:
- PALM microscopy can be used to track SMC complexes
To understand dynamic interactions between Smc5/6 and the chromatin, the authors performed single molecule tracking using photoactivated Localisation Microscopy (PALM) in live cells, which allows the capture of precise spatial information regarding the origin of the fluorescent signal (3). Briefly, when the sample is pulsed stochastically with an ultraviolet (UV) laser, the photoconvertible fluorophore mEos3 will ‘photoconvert’ from a green fluorescent signal to a red fluorescent signal. This process is repeated until all the mEos3 molecules have converted. Stochastic photoconversion of a small number of molecules at any one time means each fluorophore emission can be detected, the centre point of each ‘spot’ calculated and the information used to pinpoint the signal origin, within ~ 20nm. Here the authors fused mEos3 to the kleisin subunits of each SMC complex. Using S. pombe condensin kleisin (Cnd2; 4), as a proof-of-principle to monitor SMC complex behaviour at the chromatin in live cells and newly developed software ‘Spot-On’ (5) they tracked single molecules of Cnd2 in the nucleus and on the chromatin. Molecules bound to chromatin diffuse less than unbound molecules. This approach is known as single-particle tracking PALM (sptPALM).
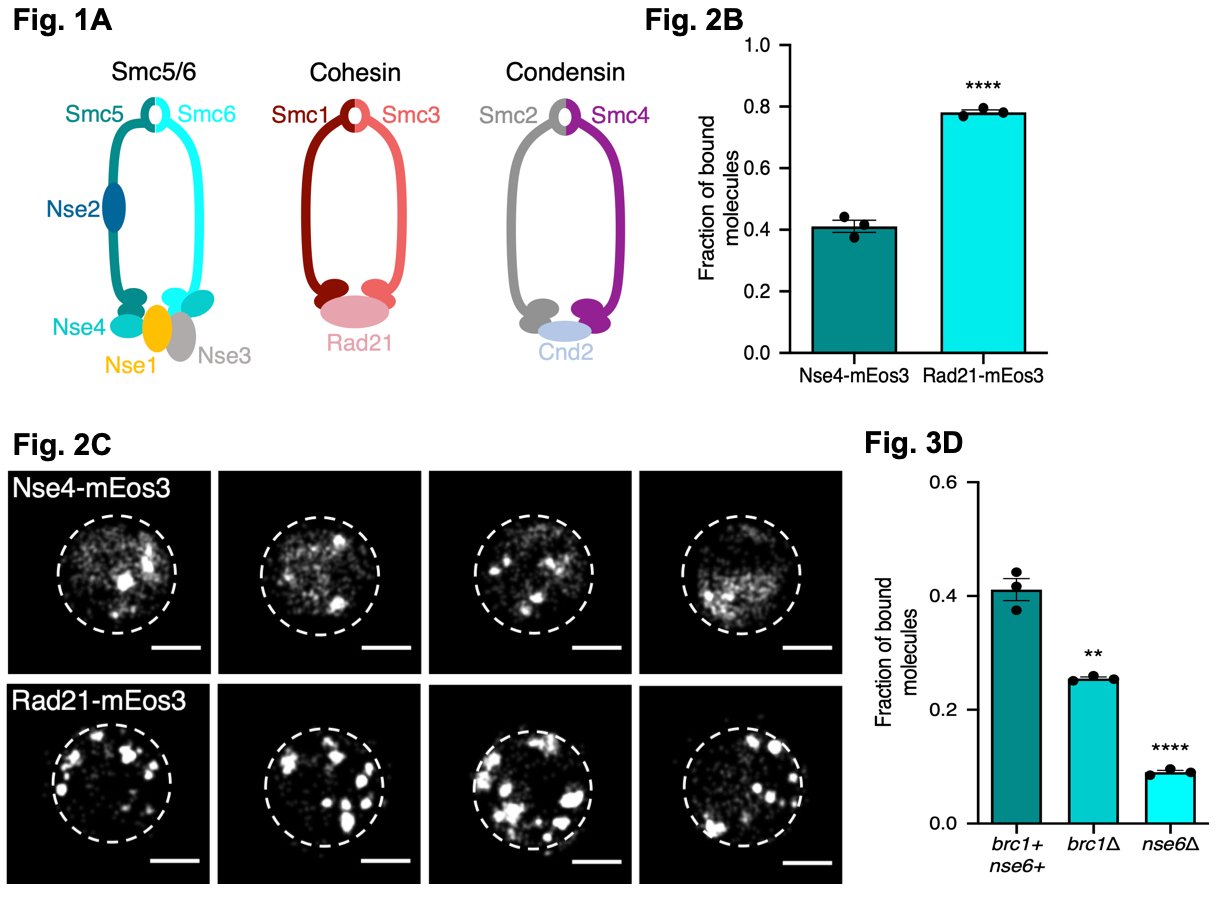
Figure 1. Fig. 1A illustrates the core composition of fission yeast SMC complexes. Fig. 2B the fraction of bound molecules of each protein to the chromatin (measured by sptPALM using the ‘Spot On’ software) for both the Smc5/6 (Nse4-mEos3) and the Cohesin complex (Rad21-mEos3). Fig. 2C sptPALM images showing the locations of Nse4-mEos3 and Rad21-mEos3 single-molecules in the nucleus (white dashed lines). Fig. 3D the fraction of bound molecules of each protein to the chromatin (measured by sptPALM using the ‘Spot On’ software) for Smc5/6 (Nse4-mEos3) in wild type cells and in cells lacking either Brc1 or Nse6. Figures made available under a CC-BY-NC-ND 4.0 International license.
- Cohesin and Smc5/6 interact differently and distinctly with chromatin
Using the above approach, the authors investigated the association of cohesin and Smc5/6 to the chromatin by fusing Nse4 (for Smc5/6) and Rad21 (for cohesin) to mEos3. Using their modelling software, the show Smc5/6 and cohesin interact differently with the chromatin, with cohesin binding more to the chromatin than Smc5/6 and the cohesin complex mostly formed nuclear foci where as Smc5/6 formed foci and showed a diffuse localisation.
- Nse6 likely loads or stabilises Smc5/6 on chromatin
To ask what factors might recruit Smc5/6 to the chromatin, the authors investigated two factors: Nse6 and Brc1. First, using sptPALM, they show Nse6 likely acts to load or stabilise the Smc5/6 complex. Next, they ask whether Nse6 or Brc1 are needed to localise Smc5/6 on the chromatin by deleting Nse6 or Brc1 in the Nse4-mEos3 cell line. Deletion of either gene disrupted the wild-type localisation of Nse4 with both genes resulting in a loss of Smc5/6 association to the chromatin. However, this loss was greatest when Nse6 was deleted supporting its role as a loader or stabiliser of Smc5/6. When cells were exposed to a genotoxin (methyl methanesulfonate; MMS) in wild-type cells, Smc5/6 was found to associate to chromatin, whereas in both deletion mutants, no chromatin association was detected (though this was independent of MMS treatment) supporting the role of Smc5/6 being loaded in response to DNA damage.
- Smc5/6 needs ATPase activity to fully associate with chromatin
Next, the authors investigated if the ATPase activity of Smc5/6 was needed for chromatin association. By disrupting the ‘arginine-finger’ region needed for ATP interactions in either Smc5 or Smc6 in the Nse4-mEos3 cell line then performing sptPALM, they reveal 1) both mutants were sensitive to replication stress exposure, with the Smc6 mutant more so, 2) both mutants show less association to the chromatin with the Smc6 mutant showing a more extreme phenotype, 3) both mutants were largely unable to recruit Smc5/6 to the chromatin in the presence of MMS treatment. In all, the ATPase activity is needed for Smc5/6 recruitment to chromatin and individually, Smc5 and Smc6 show differences in their ATP binding capacity.
- Smc5/6 interacts with ssDNA to stop chromosomes rearranging
The Smc5/6 complex can bind both ds- and ss-DNA. By reducing the ability of their Nse4-mEos3 cell line to bind dsDNA (by disrupting Nse3), they reveal the Nse3-dsDNA interaction is likely needed for Smc5/6 recruitment to chromatin. Next, they asked if the same was true for ssDNA. By mutating regions in Smc5 and Smc6 needed for ssDNA binding, they show Smc5/6-ssDNA interactions are not needed for Smc5/6 to be recruited to chromatin but under DNA damage conditions (via MMS exposure), these Smc5/6 ssDNA-binding mutants were largely unable to associate with chromatin. This suggest ssDNA may ‘keep’ Smc5/6 on the chromatin during a damage event and to bring in other factors after Smc5/6 loading to regulate repair. Next, the authors asked if Smc5/6 doesn’t need ssDNA for recruitment to chromatin, then why may it need to interact with ssDNA? Using their mutants and combining them with a previously described system in yeast for monitoring homologous recombination (HR) dynamics they investigate this. In short, when the assay is induced, an arrested replication fork occurs within a defined genomic location. Replication can only then continue once another region (ura4) is used to restart replication. This reaction is HR dependent and can result in genomic instability due to large scale genomic rearrangements (i.e a non-homologous allele is used for restart) or when the re-started replication fork makes errors as it moves. Finally, if HR fails to occur, the cells are non-viable. They reveal, the ssDNA-binding deficient mutant presented with chromosome rearrangements indicating Smc5/6 may bind ssDNA to stop these rearrangements from occurring thereby regulating HR rather than controlling it.
What I liked about this preprint:
I really enjoyed the use of sptPALM by the authors. By focusing on one technique and using it to address several questions, they reveal important findings about how Smc5/6 interacts with chromatin, including under damaging conditions. Here, they have really shown the resolution power of PALM and how it can be applied to successfully solve protein dynamics in live cells.
Questions for the authors:
- Have you performed sptPALM on Brc1 to localise this protein in the cell?
- Smc5/6 may also have important roles during S-phase. Do you plan to study the dynamics of its interaction by sptPALM within this particular stage?
- Here you have used MMS to cause DNA damage. Would you expect similar dynamics of Smc5/6 following exposure to, for example, a replication poison like Hydroxyurea, which would result in ssDNA accumulation and the stalling of DNA replication? Or another genotoxin such as phleomycin, which induces DSBs?
- You mention a possible pathway independent to Brc1 due to the weaker effect on chromatin association observed compared to Nse6 deletion. Do you think it is possible that Nse6 could instead compensate for the loss of Brc1 rather than a separate pathway operating?
References:
- Aragon, L. The Smc5/6 Complex: New and Old Functions of the Enigmatic Long-Distance Relative. Ann. Rev. Gen. 52 (2018).
- Adamus, A., Lelkes, E., Potesil, D., Ganji, S.R., Kolesar, P., Zabrady, K., Zdrahal, Z., and Palecek, J.J. Molecular insights into the architecture of the human Smc5/6 complex. JMB 13 (2020).
- Sydor, A.M., Czymmek, K.J., Puchner, E.M., and Mennella, V. Super-Resolution microscopy: from single molecules to supramolecular assemblies. Trends in Cell. Bio. 25 (2015).
- Sutani, T., Yuasa, T., Tomonaga, T., Dohmae, N., Takio, J., and Yanagida, M. Fission yeast condensing complex: essential roles of non-SMC subunits for condensation and Cdc2 phosphorylation of Cut3/SMC3. Genes Dev. 13 (1999).
- Hansen, A.S., Woringer, M., Grimm, J.B., Lavis, L.D., Tjian, R., and Darzacq, X. Robust model-based analysis of single particle tracking experiments with Spot-On. eLife (2018).
doi: https://doi.org/10.1242/prelights.22354
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the molecular biology category:
A drought stress-induced MYB transcription factor regulates pavement cell shape in leaves of European aspen (Populus tremula)
Jeny Jose
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
preLists in the molecular biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |











 (No Ratings Yet)
(No Ratings Yet)