TORC1 modulation in adipose tissue is required for organismal adaptation to hypoxia in Drosophila.
Posted on: 9 July 2018
Preprint posted on 20 June 2018
Article now published in Nature Communications at http://dx.doi.org/10.1038/s41467-019-09643-7
Hypoxia is deadly to some organisms but well-tolerated by others. New preprint sheds light on how Drosophila larvae are able to survive in low oxygen.
Selected by Sarah BowlingCategories: developmental biology
Background of the preprint
Development is a time of rapid cellular growth and proliferation. However, variabilities in food, oxygen, temperature and toxin exposure all impact these processes. For organisms such as Drosophila, these factors can vary considerably during larval development. How then do fly larvae achieve robust growth in the face of a fluctuating environment?
In response to nutrient deprivation, environmental sensing is carried out in specific Drosophila tissues such as the fat body, where the nutrient-sensing TOR pathway responds and alters whole body development to promote survival through the modulation of endocrine signalling. However, much less is known about how hypoxia is sensed and tolerated in fly development. Here, the authors aim to answer the question of how fly larvae survive in low oxygen. Since hypoxia contributes to the pathophysiology of various conditions including heart disease, obesity and cancer, gleaning information into how tissues withstand this insult could prove valuable in the treatment of human disease.
Key findings of the preprint
Firstly, the authors demonstrate that TOR signalling is rapidly repressed in response to hypoxia in fly larvae, suggesting that this pathway acts as a sensor of low oxygen levels. These changes in TOR signalling are important for fly larvae to tolerate hypoxia, since preventing TOR repression by overexpression of TOR activator Rheb markedly reduces hypoxia tolerance. Notably, the authors describe marked lethality at the pupal stage in Rheb-overexpressing larvae maintained in hypoxic conditions, a phenotype not observed in wild-type hypoxic or Rheb-overexpressing normoxic larvae.
Secondly, the preprint demonstrates that TOR signalling specifically in the fat body mediates the tolerance to hypoxia. Hyperactivation of TOR signalling in the fat body, but not other larval tissues such as the neurons and intestine, reduces hypoxic larval survival to the pupal stage by around 50%.
Finally, the authors explore the pathways altered downstream of TOR repression which confer hypoxia tolerance. They observe that hypoxia leads to an increase in both lipid droplet size (shown below) and overall lipid storage in a TOR-dependent manner. Blocking lipid droplet remodelling through knockdown of Lsd2, a key protein in lipid droplet formation, triggers high pupal lethality in hypoxic conditions. This final result illustrates that lipid droplet remodelling is vital for the survival of Drosophila larvae in low oxygen.
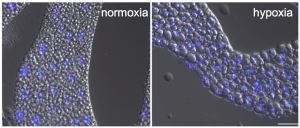
Lipid droplets in the fat body increase in size in response to hypoxia. Image reproduced with permission from Figure 6 of the preprint.
What I like about this preprint
This preprint builds on previous studies demonstrating that the fat body acts as a sensor of starvation. Taken together with previous results, this provides evidence that the fat body acts as, in the words of the authors, a ‘sentinel tissue to detect changes in environmental conditions and to buffer the internal milieu from these changes’. The preprint is therefore an excellent example of how individual projects build up over time to shape our understanding of the bigger picture. Furthermore, the results in the preprint are complimented nicely by another preprint released at a similar time (link here) which also demonstrates that hypoxia sensing occurs in the fat body of Drosophila larvae. Both studies provide valuable insight into an area of research with increasing interest: the metabolic regulation of development.
Future directions and questions for the authors
Surprisingly, previously described hypoxia sensors REDD1 and HIF1a are not important in the suppression of TOR signalling in response to low oxygen in the fly larvae. What could alternative sensors be? In the discussion the authors suggest that interplay between the mitochondria and TOR could be responsible, for example through ROS or specific metabolites. Alternatively, could the lack of effect following deletion of REDD1/HIF1a homologs be a result of compensation from other upstream components of the TOR pathway?
An interesting observation from the study was that the downstream consequences of TOR repression are different in the context of hypoxia versus nutrient starvation. Specifically, when starved of oxygen, fat body lipid droplets increase in size, whereas when starved of food, fat body lipid droplets decrease in size. Since both physiological effects are mediated through TOR signalling, it would be interesting to know how the differential downstream effects of TOR repression are elicited. Could it be a dose-dependent effect, where for example moderate vs complete repression of TOR lead to alternate lipid droplet size outcomes? Alternatively, could alternate sensors of environmental conditions, which converge on lipid signalling, be responsible? Greater understanding of the signalling events both upstream and downstream of TOR could help yield insight into these questions.
The authors show that remodelling of lipid storage is responsible for the hypoxia tolerance, although exactly how this confers protection is not known. The authors suggest two possibilities: the droplets could act as a lipid store to fuel the energy-expensive process of metamorphosis, or they could provide protection from stress. Alternatively, could lipid droplets impact on endocrine signalling and orchestrate a whole-body physiological response? This could tie in with a potential parallel mechanism for tolerating hypoxia through inter-organ communication which is explored in the previously mentioned complementary preprint. For me, this downstream aspect is the most exciting avenue for future research since this is the most likely to be exploited for therapeutic benefit in diseases where hypoxia plays a negative role.
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the developmental biology category:
Cooperation between cortical and cytoplasmic forces shapes planar 4-cell stage embryos
Corentin Mollier, Shivani Dharmadhikari
A drought stress-induced MYB transcription factor regulates pavement cell shape in leaves of European aspen (Populus tremula)
Jeny Jose
Cross Sectional and Longitudinal Imaging Reveals Spatiotemporal Divergence in Morphogenesis and Cell Lineage Specification between in-vivo and in-vitro Mouse Embryo during Pre- and Peri-implantation
Heather Pollington
preLists in the developmental biology category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
BSDB/GenSoc Spring Meeting 2024
A list of preprints highlighted at the British Society for Developmental Biology and Genetics Society joint Spring meeting 2024 at Warwick, UK.
| List by | Joyce Yu, Katherine Brown |
GfE/ DSDB meeting 2024
This preList highlights the preprints discussed at the 2024 joint German and Dutch developmental biology societies meeting that took place in March 2024 in Osnabrück, Germany.
| List by | Joyce Yu |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
2nd Conference of the Visegrád Group Society for Developmental Biology
Preprints from the 2nd Conference of the Visegrád Group Society for Developmental Biology (2-5 September, 2021, Szeged, Hungary)
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Society for Developmental Biology 79th Annual Meeting
Preprints at SDB 2020
| List by | Irepan Salvador-Martinez, Martin Estermann |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EDBC Alicante 2019
Preprints presented at the European Developmental Biology Congress (EDBC) in Alicante, October 23-26 2019.
| List by | Sergio Menchero et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |
Pattern formation during development
The aim of this preList is to integrate results about the mechanisms that govern patterning during development, from genes implicated in the processes to theoritical models of pattern formation in nature.
| List by | Alexa Sadier |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |











 (No Ratings Yet)
(No Ratings Yet)