VAP spatially stabilizes dendritic mitochondria to locally fuel synaptic plasticity
Posted on: 10 February 2023 , updated on: 13 February 2023
Preprint posted on 18 January 2023
A novel player regulating mitochondrial stabilization in the dendrites: A proteomic screen uncovers the role of VAP in tethering mitochondria for continuous fuel supply during synaptic activity.
Selected by Kritika MehtaCategories: biochemistry, neuroscience
Background:
Local information processing in neuronal synapses is energy-intensive and requires a continuous supply of ATP for incurring long-term changes at the synapses1. Making up only 2% of our total body weight, a human brain utilizes about 20% of the body’s total oxygen for its optimal functioning1,2. As shown previously, inhibiting mitochondrial activity blocks protein synthesis-dependent synaptic plasticity3. This observation means that a stable mitochondrial compartment is required at the dendrites to fuel local changes at the synapses in response to synaptic activity.
While the players involved in docking the mitochondria in axonal terminals have been identified and well characterized, the mechanistic basis of mitochondrial tethering and stabilization within neuronal dendrites remains unknown. To address this knowledge gap, the authors of this preprint screened for proteins that exist close to mitochondria and interact with actin and the microtubule cytoskeleton.
Using a knockdown and imaging approach in hippocampal neurons, the authors were able to hone in on the role of the VAP protein – already known to be involved in several neurological diseases4. The authors discovered that the VAPB paralog is particularly localized only to the dendritic compartments. Depletion of VAP from neurons affected the structural plasticity in LTP-induced spines. These findings point to VAP being a major mitochondrial stabilizer near synapses.
Key findings:
To identify the major players in tethering dendritic mitochondria, the authors employed a screening strategy using the APEX tag, which can biotinylate proteins in its vicinity. The APEX tag was targeted to the mitochondrial outer membrane (OMM) and expressed in hippocampal neurons. The captured proteins were identified by mass spectrometry, and the resulting list was filtered for proteins with known interactions with actin and the microtubule cytoskeleton. Eventually, the authors narrowed down to a list of 8 proteins that could be potential players in regulating mitochondrial stability in the dendritic compartments and hence fuel local synaptic plasticity events.
To understand the role of the 8 identified proteins in ensuring dendritic mitochondrial stability and synaptic plasticity, the authors knocked down each of these proteins in hippocampal neurons and measured the actin-mitochondrial interaction in dendrites using Fis1-mCherry and Fis1-Lifeact-GFP. In all knockdown neurons, actin-mitochondrial interaction was significantly reduced in dendrites while unaffected in axons, which suggested a role of these proteins in mitochondrial tethering exclusively to dendritic actin.
To further understand the mechanism by which mitochondrial compartments are stabilized in the dendrites, the authors narrowed their list to three proteins: SNCA, SRGAP2, and VAP. They monitored mitochondrial stability when these proteins were knocked down in hippocampal neurons using photoactivated fluorescence of mitochondria. Knockdown of all three proteins reduced the mitochondrial length at the dendrites. However, of all three knockdowns, only dendrites with VAP knockdown showed a significant decrease in photoactivated mitochondrial compartment fluorescence corresponding to destabilized mitochondrial compartments.
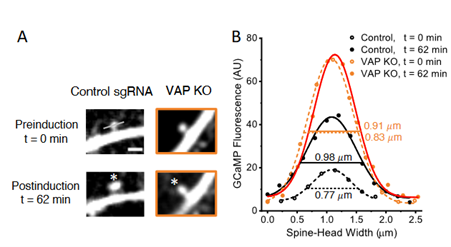
Figure 1 (original fig 6A-B) measuring spine-head width and GCaMP fluorescence before and after synaptic plasticity induction in control and VAP KO hippocampal neurons.
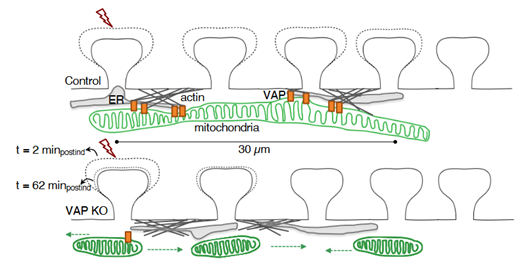
Figure 2 (original fig 6F) Schematic showing VAP as a mitochondrial stabilizer within dendrites supporting synaptic plasticity.
Since VAP is associated with various neurological diseases, the authors then aimed to identify the localization of both paralogs of VAP, VAPA and VAPB, with respect to mitochondria within dendrites and axons. Interestingly, VAPB was specifically enriched near dendritic mitochondria, while VAPA was enriched near dendritic and axonal mitochondria. The specific enrichment of VAPB near dendritic mitochondria made the authors wonder whether VAP protein’s paralog B could be a major stabilizer of dendritic mitochondria and locally support synaptic plasticity. To test this, the authors induced single-spine plasticity using two-photon glutamate uncaging. Spine size was measured in hippocampal neurons with and without VAP knockdown. Interestingly, VAP depletion did not affect the early stages of synaptic plasticity (t=12 mins). However, at later time points (t=22 mins to t=62 mins), spines lacking VAP were unable to retain structural plasticity (increased spine size) (figure 1). Additionally, the ability to exhibit clustered plasticity was also lost in VAP-depleted spines. The authors finally demonstrate a model where VAP tethers and stabilizes dendritic mitochondria, and this spatial stability enables continuous ATP supply via oxidative phosphorylation during synaptic plasticity (see figure 2 above).
What I liked about the preprint:
How a neuron’s intrinsic machinery meets the extensive energy requirements in different compartments is intriguing to me. It is also interesting how different mechanisms exist for mitochondrial stabilization in different neuronal compartments, as loss of each could have diverse but detrimental effects on brain functioning and disease progression. This preprint reflects a significant advancement in our understanding by identifying a critical player facilitating the crucial ATP supply required for long-term synaptic plasticity.
The authors’ approach to identifying new, relevant proteins is impressive to me as this strategy allowed them to screen in a more physiologically relevant model.
Questions to authors:
- It is my understanding that VAP brings mitochondria and dendritic actin together. Has domain specificity of VAP for actin and the ER been identified? If yes, is the remaining portion of the VAP protein sufficient for mitochondrial tethering?
- Could VAP-associated mitochondria have enhanced stability via the preclusion of the fission-fusion machinery?
- As a mitochondrial stabilizer, could VAP be more enriched in active dendrites in comparison to inactive dendrites?
Future Directions:
Studies in the future should focus on validating the role of VAP in stabilizing dendritic mitochondria. Since VAP is enriched in dendrites, it will be interesting to see if only VAP-positive dendritic segments carry mitochondria or if mitochondrial tethering only happens after synaptic activity. Other relevant proteins identified in this preprint should also be explored further for their contribution to dendritic mitochondrial tethering and stabilization.
References:
- Mink, J. W., Blumenschine, R. J. & Adams, D. B. Ratio of central nervous system to body metabolism in vertebrates: its constancy and functional basis. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 241, R203–R212 (1981).
- Harris, J. J., Jolivet, R. & Attwell, D. Synaptic Energy Use and Supply. Neuron 75, 762–777 (2012).
- Rangaraju, V., Lauterbach, M. & Schuman, E. M. Spatially Stable Mitochondrial Compartments Fuel Local Translation during Plasticity. Cell 176, 73-84.e15 (2019).
- Nishimura, A. L. et al. A Mutation in the Vesicle-Trafficking Protein VAPB Causes Late-Onset Spinal Muscular Atrophy and Amyotrophic Lateral Sclerosis. The American Journal of Human Genetics 75, 822–831 (2004).
doi: https://doi.org/10.1242/prelights.33633
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the biochemistry category:
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Enzymatic bromination of native peptides for late-stage structural diversification via Suzuki-Miyaura coupling
Zhang-He Goh
Mitochondrial Fatty Acid Oxidation is Stimulated by Red Light Irradiation
Rickson Ribeiro, Marcus Oliveira
Also in the neuroscience category:
Imaging cellular activity simultaneously across all organs of a vertebrate reveals body-wide circuits
Muhammed Sinan Malik
Post-translational Tuning of Human Cortical Progenitor Neuronal Output
Jawdat Sandakly
Maturation of the glymphatic system confers innate resistance of the brain to Zika virus infection
Jimeng Li
preLists in the biochemistry category:
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
Community-driven preList – Immunology
In this community-driven preList, a group of preLighters, with expertise in different areas of immunology have worked together to create this preprint reading list.
| List by | Felipe Del Valle Batalla et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
Peer Review in Biomedical Sciences
Communication of scientific knowledge has changed dramatically in recent decades and the public perception of scientific discoveries depends on the peer review process of articles published in scientific journals. Preprints are key vehicles for the dissemination of scientific discoveries, but they are still not properly recognized by the scientific community since peer review is very limited. On the other hand, peer review is very heterogeneous and a fundamental aspect to improve it is to train young scientists on how to think critically and how to evaluate scientific knowledge in a professional way. Thus, this course aims to: i) train students on how to perform peer review of scientific manuscripts in a professional manner; ii) develop students' critical thinking; iii) contribute to the appreciation of preprints as important vehicles for the dissemination of scientific knowledge without restrictions; iv) contribute to the development of students' curricula, as their opinions will be published and indexed on the preLights platform. The evaluations will be based on qualitative analyses of the oral presentations of preprints in the field of biomedical sciences deposited in the bioRxiv server, of the critical reports written by the students, as well as of the participation of the students during the preprints discussions.
| List by | Marcus Oliveira et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
20th “Genetics Workshops in Hungary”, Szeged (25th, September)
In this annual conference, Hungarian geneticists, biochemists and biotechnologists presented their works. Link: http://group.szbk.u-szeged.hu/minikonf/archive/prg2021.pdf
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Also in the neuroscience category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |











 (1 votes)
(1 votes)