Embryological manipulation to probe early evo-devo in the fish Astyanax mexicanus
Posted on: 26 November 2020 , updated on: 1 January 2021
Preprint posted on 7 October 2020
Article now published in Frontiers in Cell and Developmental Biology at http://dx.doi.org/10.3389/fcell.2021.667296
Gastruloids, pescoids, caveoids, surfoids….In vitro embryonic models to study evo-eco-devo. New experimental approaches to cavefish development.
Selected by Paul Gerald L. Sanchez and Stefano VianelloCategories: developmental biology
Overview of the research
The mexican cavefish (Astyanax mexicanus) is a freshwater fish living in the rivers and caves bordering the Mexican Gulf. Uniquely, members of this species exist in two very different forms (“eco-morphotypes”) (See Figure 1). The surface morph lives in rivers, is sighted and pigmented. The cave morph lives deep into dark caves, is blind, depigmented, but compensates for the loss of sight by a highly developed olfactory system. Clearly, each form of this species is highly adapted to its environment, but how is their same genetic identity executed into such different body plans during development? What are the developmental differences that turn an Astyanax embryo into a “surface” fish or a “cave” one?
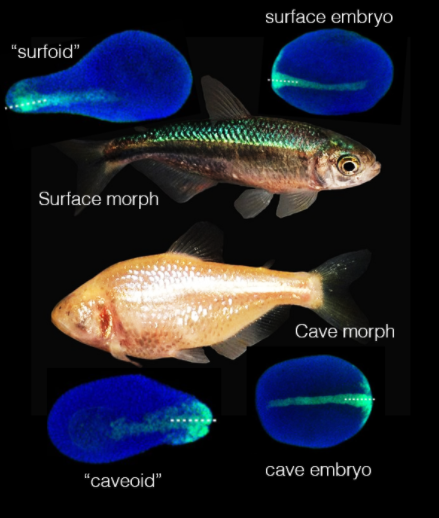
Figure 1: the two Astyanax morphs. Morph picture is from wikimedia commons (public domain). Embryos and pescoids are reused with permission from preprint Fig.2.
Previous work in this species has identified that there are differences in the maternal determinants provided by each mother morph during egg formation, and deposited in the yolk. (Torres-Paz, et al; 2019, Ma, et al; 2018). Eggs from cave mothers are enriched in ventralising signals compared to surface eggs, suggesting that some of the morphological differences observed come maternally rather than from the embryo itself (maternal genetic effect). How to test the extent of such maternal contributions?
The authors turn to experimental and synthetic embryology, and describe the successful application, to the mexican cavefish, of two techniques that both probe the intrinsic developmental programmes of surface and cave embryonic cells. Accordingly, the authors describe the procedure to generate cave-surface chimeric embryos: i.e. unique embryos made from cells of both morph types. Secondly, the authors use cavefish embryonic cells to generate gastruloids, a novel in vitro system widely employed to dissect the role of embryonic versus extraembryonic cues (see the preList on the topic).
Key Results
Chimeric embryos
The authors overcome significant hurdles related to Astyanax reproductive biology, and describe a protocol to generate stage-matched chimeric embryos between the two morphs: that is, to take cells from a surface fish embryo and incorporate them into a cave morph (and vice versa). Preliminary results show that cells from different morphs are able to survive together and to contribute to embryonic tissues based on the time of transplantation (restriction of potential as time progresses). It would be interesting to follow up on whether these cells initiate independent developmental programmes typical of their morph of origin, or if either developmental programme prevails over the other.
Ecotype-specific pescoids (fish gastruloids)
By applying a recently described protocol to generate fish gastruloids (“pescoids”, Fulton et al; 2020) the authors generate “surfoids” (pescoids made from surface fish embryonic cells), and “caveoids” (pescoids made from cave fish embryonic cells) (See Figure 2). By showing that cells from Astyanax embryos also aggregate and elongate in vitro by self organisation, in the absence of yolk, the authors expand on the now growing collection of species whose embryonic cells have been shown to recapitulate features of normal development in vitro (human, mouse, zebrafish). The rationale of this approach is particularly intriguing: if you take Astyanax cells away from the yolk and maternal factors and make them assemble as pescoids, do they still “know” they should execute “surface” and “cave” programmes? For now, the authors do not report any notable difference between the behaviour of the two pescoid morph types, at least with respect to the readout analysed in this report. Incidentally, staining for the marker no tail (ntl, equivalent of the mouse Brachyury) shows that mesoderm tissue internalises, at the site equivalent to the blastopore in an intact embryo. An observation that enriches current knowledge about how pescoids develop.
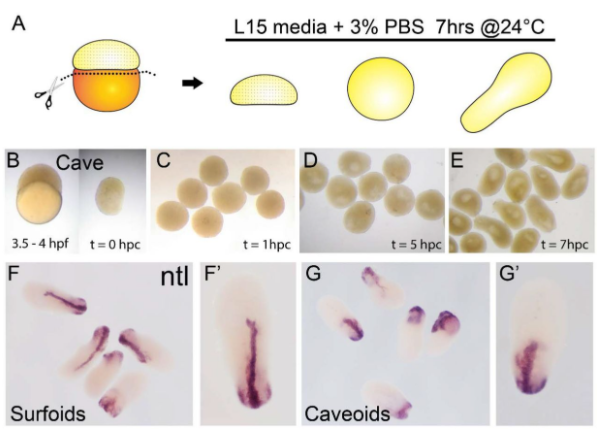
Figure 2: Pescoid generation. Surfoids and Caveoids generated by in vitro culture of Astyanax embryonic cells away from the yolk. Purple: staining for the mesoderm marker no tail (ntl) (reused with permission from preprint Fig.1)
Significance
This preprint describes early steps towards the novel application of experimental embryology techniques to a very interesting case study: the mexican cavefish. The recourse to the gastruloid/pescoid approach is extremely interesting, because here is a system where cells with the same genetic identity give rise to embryos with drastically different morphology and adult structures. Gastruloids, where embryonic cells are left to their own means without any other developmental cue, are an excellent system to tease out the relative contribution of embryonic and maternal inputs to development. If features of cave and surface morphology are instructed by maternal cues, one would expect caveoids and surfoids not to be able to develop such features. We are left wondering what will come next from the application of the two techniques described in this paper.
Questions to the authors:
- One of the major differences of cave morphs is their degenerate eye and the loss of vision. In the preprint you indeed mention the availability of an Astyanax eye reporter that would allow to track the fate of these cells during development. Could you elaborate more on this project? Do you expect cave cells to be rescued in a cave-surface chimaera?
- On the same topic, many notable differences of cave morphs relate to facial anatomy and anterior neural structures. These are also the structures that have traditionally been missing in gastruloids. Do you think this will represent a significant obstacle to the pescoid approach?
- Pescoids have originally been developed with zebrafish cells. What is known about the evolutionary divergence between cavefish and zebrafish? Do you believe pescoids could be generated from any fish species?
- A technical question: why are cave morphs maintained at a 12:12 light-dark cycle? Do they not usually live in dark caves all day long? Would your observations be different if the fish were maintained in a longer dark phase?
References
- Torres-Paz, J., Leclercq, J., & Rétaux, S. 2019. Maternally-regulated gastrulation as a source of variation contributing to cavefish forebrain evolution. ELife, 8. https://doi.org/10.7554/eLife.50160
- Ma, Li, et al. “Maternal genetic effects in Astyanax cavefish development.” Developmental biology 441.2 (2018): 209-220.
- Fulton, Timothy, et al. “Axis Specification in Zebrafish Is Robust to Cell Mixing and Reveals a Regulation of Pattern Formation by Morphogenesis.” Current Biology 30.15 (2020): 2984-2994
doi: https://doi.org/10.1242/prelights.25860
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the developmental biology category:
Tissue mechanics and systemic signaling safeguard epithelial tissue against spindle misorientation
Ruoheng Li
Human pluripotent stem cell-derived macrophages modify development of human kidney organoids
Theodora Stougiannou
Junctional Heterogeneity Shapes Epithelial Morphospace
Bhaval Parmar
preLists in the developmental biology category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
BSDB/GenSoc Spring Meeting 2024
A list of preprints highlighted at the British Society for Developmental Biology and Genetics Society joint Spring meeting 2024 at Warwick, UK.
| List by | Joyce Yu, Katherine Brown |
GfE/ DSDB meeting 2024
This preList highlights the preprints discussed at the 2024 joint German and Dutch developmental biology societies meeting that took place in March 2024 in Osnabrück, Germany.
| List by | Joyce Yu |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
2nd Conference of the Visegrád Group Society for Developmental Biology
Preprints from the 2nd Conference of the Visegrád Group Society for Developmental Biology (2-5 September, 2021, Szeged, Hungary)
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Society for Developmental Biology 79th Annual Meeting
Preprints at SDB 2020
| List by | Irepan Salvador-Martinez, Martin Estermann |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EDBC Alicante 2019
Preprints presented at the European Developmental Biology Congress (EDBC) in Alicante, October 23-26 2019.
| List by | Sergio Menchero et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |
Pattern formation during development
The aim of this preList is to integrate results about the mechanisms that govern patterning during development, from genes implicated in the processes to theoritical models of pattern formation in nature.
| List by | Alexa Sadier |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |











 (No Ratings Yet)
(No Ratings Yet)